Synthesis method of 2-(2-methoxy-4-nitrophenyl)-3-(4-nitrophenyl)-5-(2,4-disulfophenyl)-2H-tetrazole monosodium salt
A technology of disulfobenzene and a synthesis method, applied in directions such as organic chemistry, can solve the problems of low total yield, high price, high toxicity, etc., and achieve the effects of easy post-processing, simple and safe operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
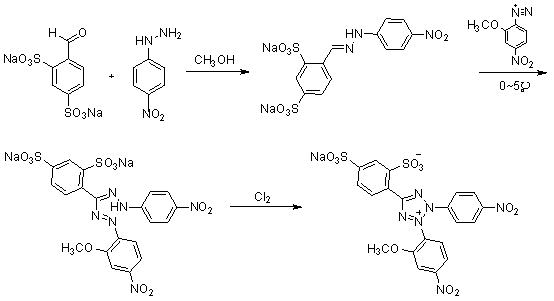
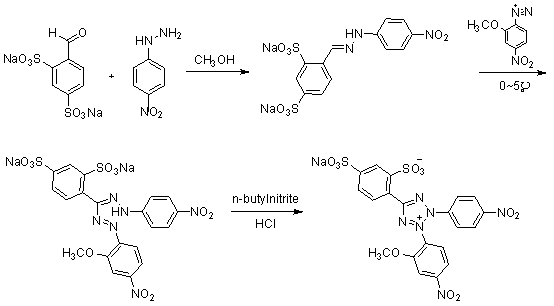
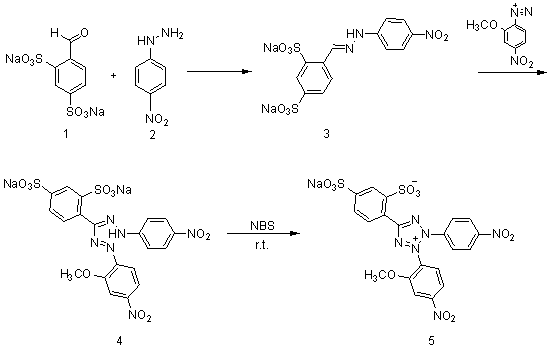
[0027] 1. Compound 3 preparation of
[0028] take compound 2 ( 4.94g, 0.0322mol ) into a 500ml three-neck flask, add 150ml of methanol, heat and stir until reflux and dissolve. Cool to 50°C, slowly add compound 1 (10.0g, 0.0322mol). Keep the temperature at 50°C and continue to stir for 2h. Suction filtration under reduced pressure, washing with a small amount of methanol to obtain the compound 3 ( 11.065g, 0.0249mol ), yield 77.4%.
[0029] 1 H-NMR ( 400MHz, D 2 O) δ H : 6.83( d, 2H, J = 9Hz ), 7.65( dd, 1H, J = 8Hz, 1Hz), 7.81( d, 2H, J = 9Hz ), 7.93( d, 1H, J = 8Hz ), 8.03( d, 1H, J = 1Hz ), 8.31( s, 1H).
[0030] 2. Preparation of 4-nitro-2-methoxydiazonium salt
[0031] Take 2-methoxy-4-nitroaniline (3.7g, 0.0220mol) in a 50ml single-necked bottle, put it in an ice bath at -10~0°C, add 6.67ml of concentrated hydrochloric acid and 15ml of hydrochloric acid solution diluted with water. Stir for 5min. Then a solution obtained by dissolving (1.64g, 0.0237...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com