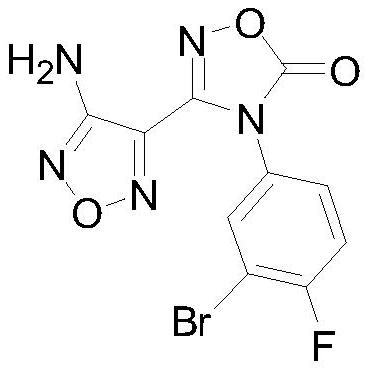
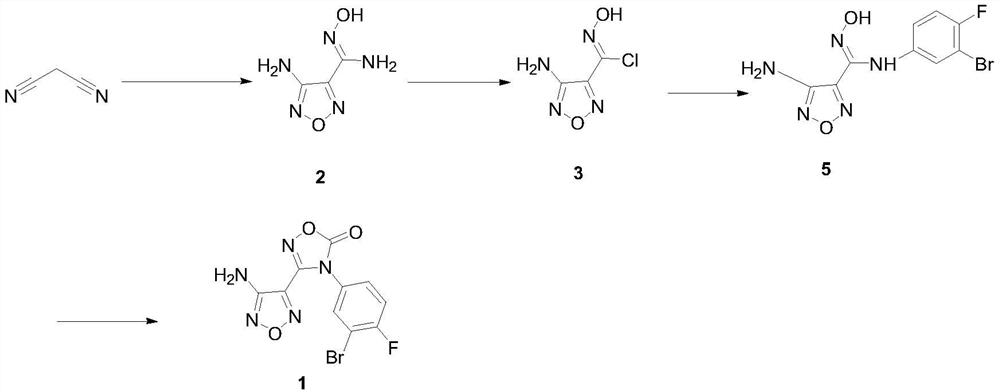
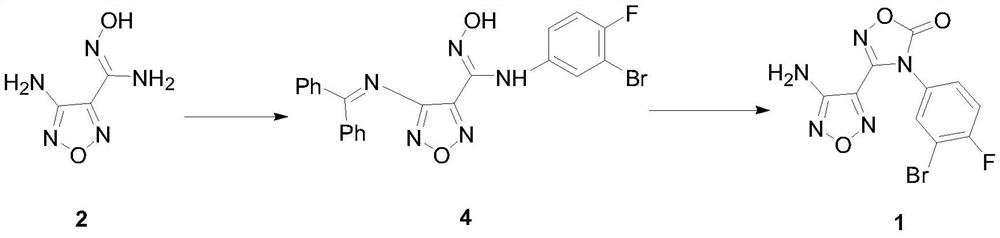
A kind of preparation method of intermediate of heterocyclic urea indoleamine-2,3-dioxygenase inhibitor
A technology of dioxygenase and indoleamine, which is applied in the intermediate of heterocyclic urea indoleamine-2,3-dioxygenase inhibitor, 3--4--1,2,4-oxadi In the field of preparation of oxazol-5-one, it can solve the problems that impurities affect the product purity, the product cost is difficult to reduce, and the reaction selectivity is poor, so as to achieve the effect of low cost, easy operation and improved selectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Preparation of 3-Amino-4-Aminoximidofurazan Intermediate 2
[0030] In a 1L three-neck flask, dissolve malononitrile (33.0g, 0.5mol) in 2mol / L hydrochloric acid (200mL), and add NaNO2 (69.0g, 1.0mol) in water (100mL) dropwise at a temperature of 0-15°C , dropwise, stirred at room temperature for 2 hours, cooled to 0°C, added dropwise a solution of hydroxylamine hydrochloride (104.2g, 1.5mol) dissolved in water (75mL), stirred at room temperature for 30 minutes after the addition, and slowly raised the temperature to reflux and stirred for 2 hours. Cool to below 20°C and adjust pH to 7-8 with 10mol / L sodium hydroxide solution, stir at 0-5°C for 1 hour, filter to obtain 62.2g of white powder, yield 87.2%, HPLC purity 98.3%, m.p.192-193 ℃, MS:144.0[M+H] + .
[0031] Preparation of 4-diphenylmethyleneamino-N-(3-bromo-4-fluorophenyl)-N’-hydroxy-1,2,5-oxadiazole-3-carboxamidine intermediate 4
[0032] Add benzophenone (79.3g, 0.435mol), trimethyl orthoformate (69.2g, 0.652...
Embodiment 2
[0036] Preparation of 3-Amino-4-Aminoximidofurazan Intermediate 2
[0037]In a 1L three-neck flask, dissolve malononitrile (33.0g, 0.5mol) in 4mol / L hydrochloric acid (100mL), and add NaNO2 (51.8g, 0.75mol) in water (150mL) dropwise at a temperature of 0-15°C , dropwise, stirred at room temperature for 2 hours, cooled to 0°C, added dropwise a solution of hydroxylamine hydrochloride (69.5g, 1.0mol) dissolved in water (50mL), stirred at room temperature for 30 minutes after the addition, slowly warmed to reflux and stirred for 2 hours, Cool to below 20°C and adjust pH to 7-8 with 10mol / L sodium hydroxide solution, stir at 0-5°C for 1 hour, filter to obtain 60.2g of white powder, yield 84.5%, HPLC purity 98.2%, m.p.192-193 ℃.
[0038] Preparation of 4-diphenylmethyleneamino-N-(3-bromo-4-fluorophenyl)-N’-hydroxy-1,2,5-oxadiazole-3-carboxamidine intermediate 4
[0039] Add benzophenone (92.2g, 0.506mol), triethyl orthoformate (81.3g, 0.549mol), p-toluenesulfonic acid (0.73g, 4.2m...
Embodiment 3
[0043] Preparation of 3-Amino-4-Aminoximidofurazan Intermediate 2
[0044] In a 1L three-neck flask, dissolve malononitrile (33.0g, 0.5mol) in 6mol / L hydrochloric acid (40mL), and add NaNO2 (69.0g, 1.0mol) in water (100mL) dropwise at a temperature of 0-15°C , dropwise, stirred at room temperature for 2 hours, cooled to 0°C, added dropwise a solution of hydroxylamine hydrochloride (104.2g, 1.5mol) dissolved in water (75mL), stirred at room temperature for 30 minutes after the addition, and slowly raised the temperature to reflux and stirred for 2 hours. Cool to below 20°C, adjust pH to 7-8 with 10mol / L sodium hydroxide solution, stir at 0-5°C for 1 hour, filter to obtain 62.4g of white powder, yield 87.5%, HPLC purity 98.0%, m.p.192-193 ℃.
[0045] Preparation of 4-diphenylmethyleneamino-N-(3-bromo-4-fluorophenyl)-N’-hydroxy-1,2,5-oxadiazole-3-carboxamidine intermediate 4
[0046] Add benzophenone (79.8g, 0.438mol), trimethyl orthoformate (69.6g, 0.656mol), p-toluenesulfonic...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com