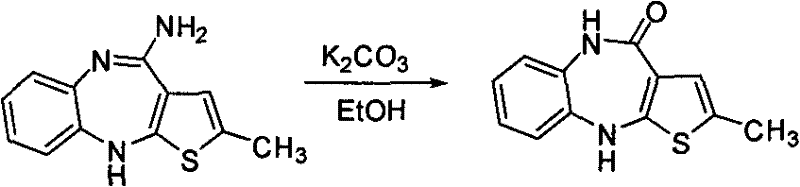Patents
Literature
486 results about "Malononitrile" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Malononitrile, also propanedinitrile or malonodinitrile, is a nitrile with the formula CH₂(CN)₂. Malononitrile is relatively acidic, with a pKₐ of 11 in water. In related chemistry, malononitrile is a suitable starting material for the Gewald reaction, where the nitrile condenses with a ketone or aldehyde in the presence of elemental sulfur and a base to produce a 2-aminothiophene.
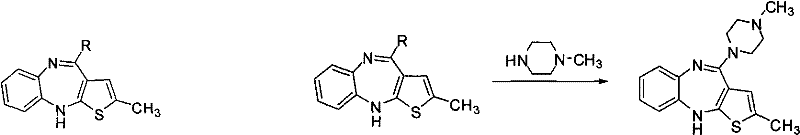
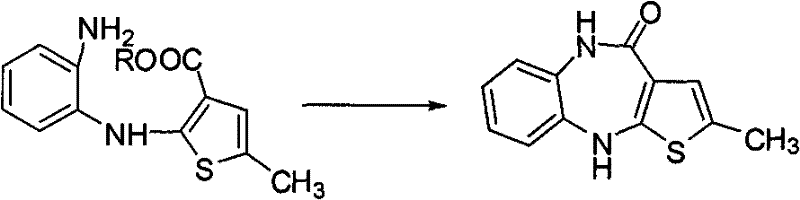
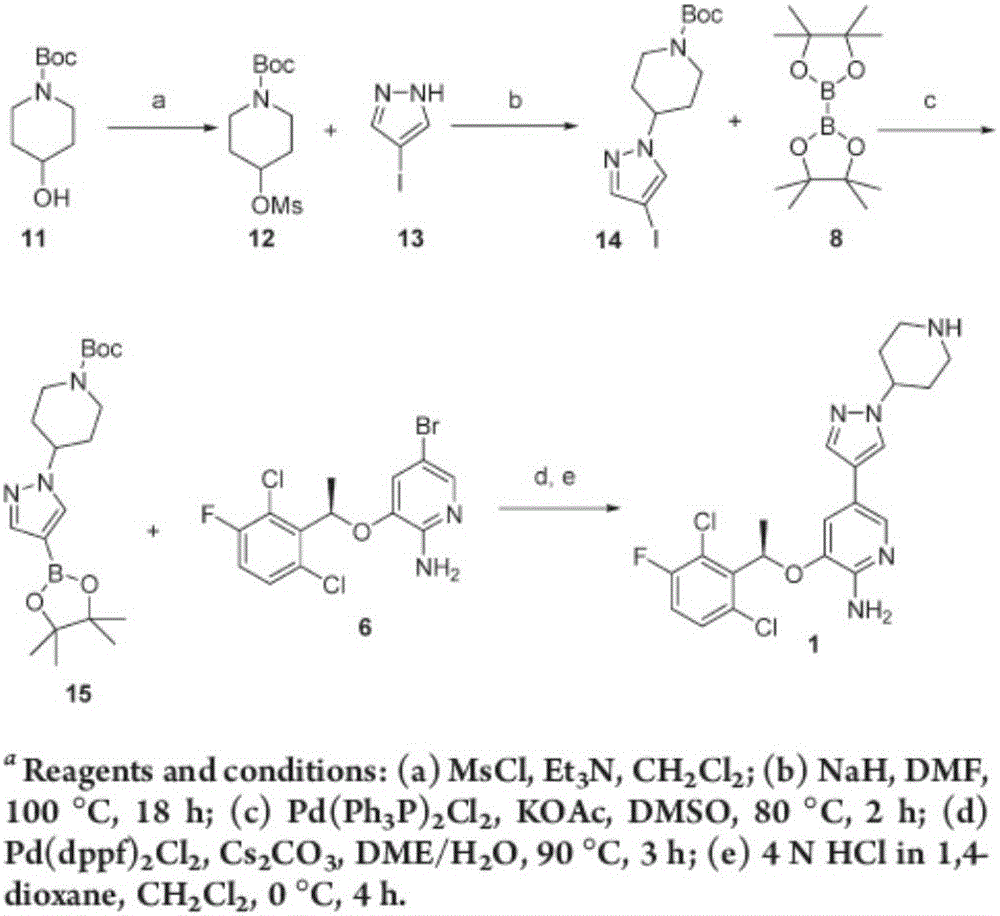
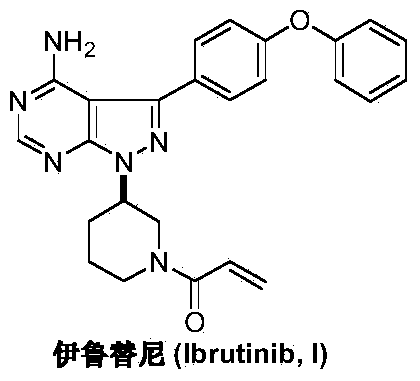
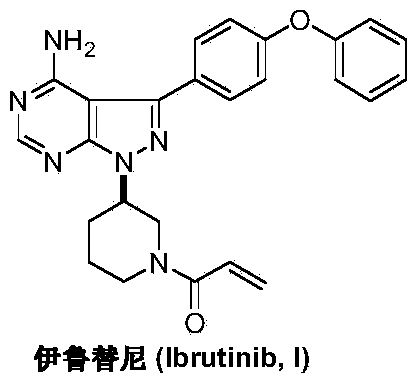
Preparation method of Ibrutinib
ActiveCN103626774AEase of industrial productionRaw materials are easy to getOrganic chemistryCyanideBenzoyl chloride
The invention discloses a preparation method of Ibrutinib (I). The preparation method comprises the following steps: performing condensation and methyl oxidization reaction on 4-phenoxyl benzoyl chloride (II) serving as a raw material and methylene cyanide and dimethyl sulfate to generate 4-phenoxyl phenyl (methoxyl) ethenylidene dicyan methane (III); performing pyrazol cyclization reaction between the intermediate (III) and 1-(3R-diazanyl-1-piperidyl)-2-propylene-1-ketone(IV) to obtain 1-[(3R)-[3-(4-phenoxyl phenyl)-4-nitrile-5-amino-1H-pyrazolyl]-1-piperidyl]-2-propylene-1-ketone(V). The pyrimidine cyclization reaction between the intermediate (V) and a cyclization agent is performed to prepare the Ibrutinib (I). The preparation method has easily-available raw materials, and is simple in process, economical and environment-friendly, and suitable for industrial production.
Owner:TONGLING WANGYANTANG BIOTECHNOLOGY CO LTD
Preparation method of pinoxaden
InactiveCN106928253AReduce pollutionEmission reductionOrganic chemistrySandmeyer reactionMethylaniline
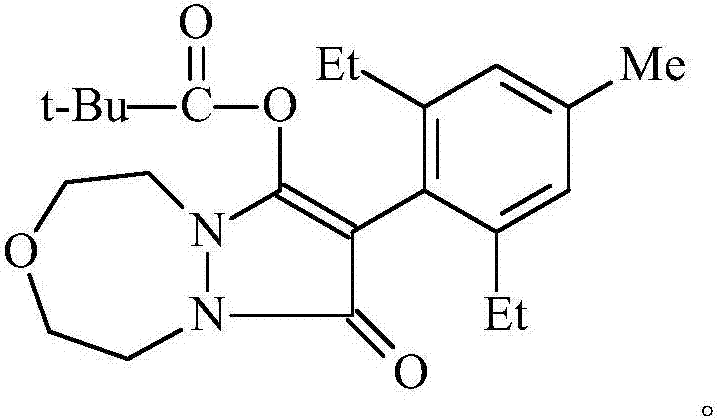
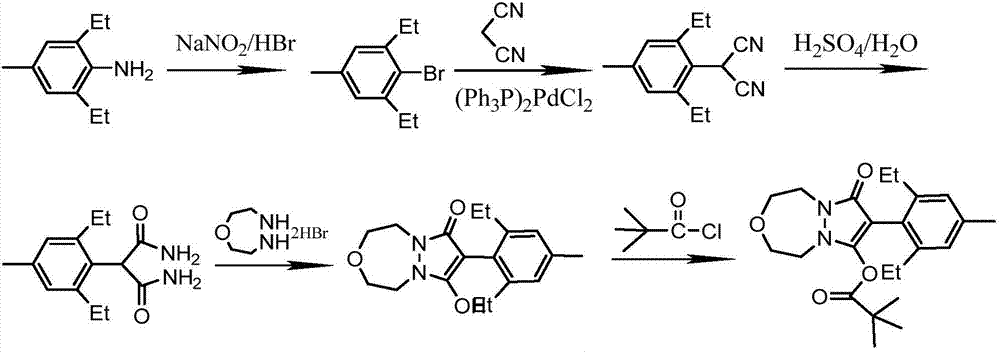
The invention relates to a method for preparing pinoxaden. The steps are as follows: 2,6-diethyl-4-methylaniline undergoes a sandmeyer reaction to obtain 2,6-diethyl-4-methylbromobenzene, and then reacts with Malononitrile is coupled under the catalysis of copper iodide to obtain 2,6-bis-ethyl-4-methylphenylmalononitrile, and finally hydrolyzed in hydrogen peroxide solution to obtain 2,6-diethyl-4 -Methylphenylmalonamide; subsequent reaction with [1,4,5]-oxadiazepine dihydrobromide in the presence of triethylamine gives 8-(2,6-diethyl- 4-methylbenzene)tetrahydropyrazole[1,2d][1,4,5]-oxadiazepine-7,9-dione, preferably reacted with pivaloyl chloride to obtain pinoxaden ester. The present invention uses low-priced catalysts to reduce production costs. In addition, hydrogen peroxide-alkali is used as the hydrolysis system in the preparation process, which greatly reduces environmental pollution.
Owner:WUHAN INSTITUTE OF TECHNOLOGY
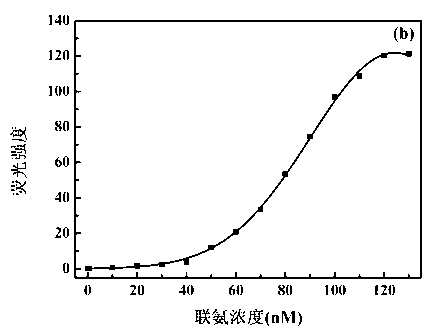
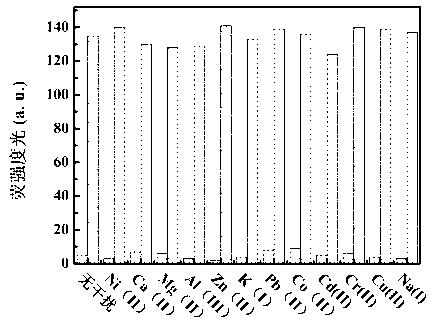
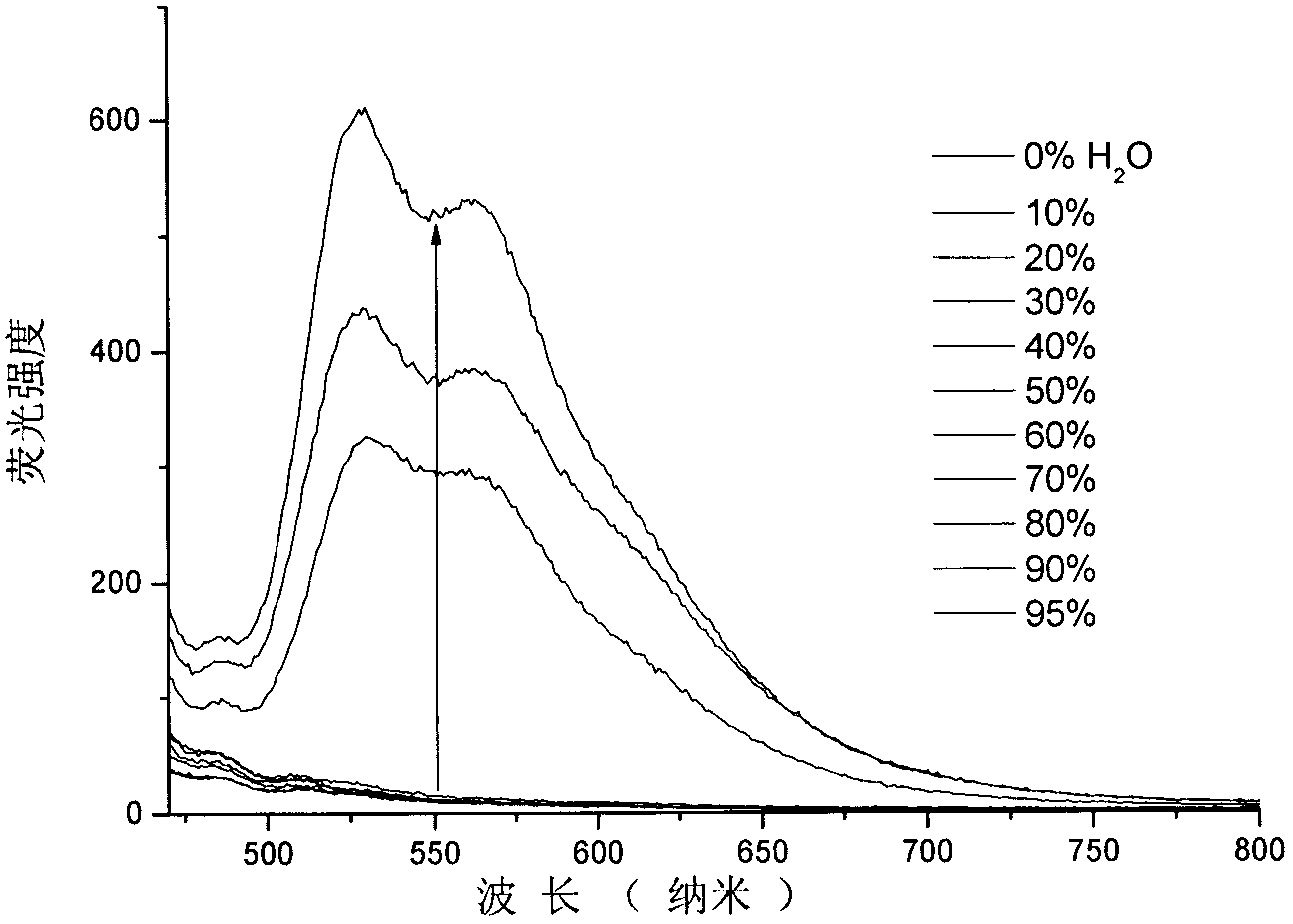
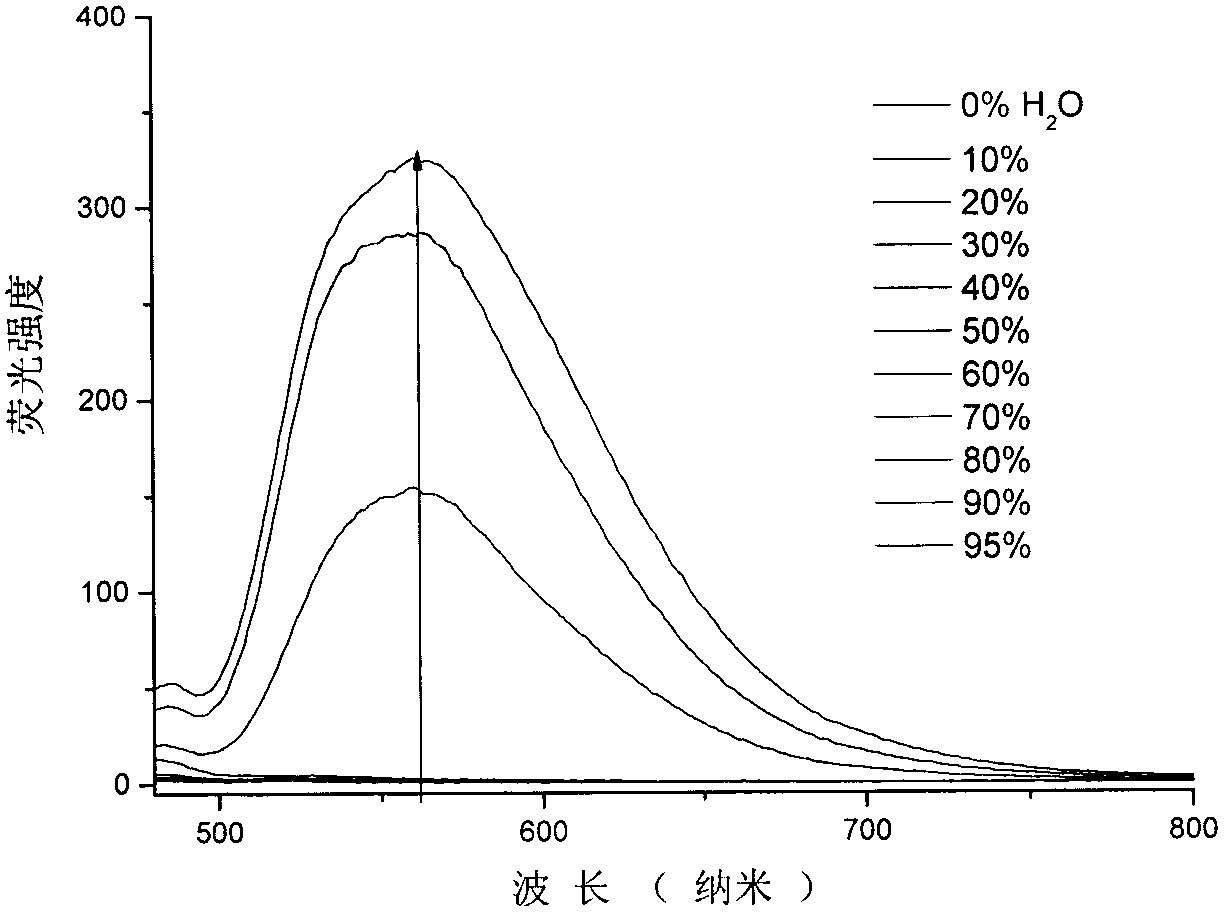
Benzothiazole-cyanophenyl compound serving as hydrazine fluorescence probe as well as preparation method and application method of benzothiazole-cyanophenyl compound
ActiveCN103214428ASimple preparation processConjugate plane largeOrganic chemistryFluorescence/phosphorescenceIndustrial waste waterStructural formula
The invention discloses a benzothiazole-cyanophenyl compound serving as a hydrazine fluorescence probe. The benzothiazole-cyanophenyl compound has a structural formula as shown in (I); the compound is prepared by performing cyclodehydration with bromobenzaldehyde and 2-amino-4-chloro thiophenol serving as the raw materials, then performing coupled reaction in order to connect with a bromobenzaldehyde derivate, and finally performing Knoevenagel reaction with malononitrile. The benzothiazole-cyanophenyl compound has the advantages that the raw materials are low in price and easy to gain, the synthetic route is simple, and the yield is relatively high; rigid structures such as benzothiazole and phenylacetylene groups are introduced into such a fluorescence probe, thus high fluorescence quantum efficiency is realized, and relatively high thermal stability and dissolubility are brought. The probe adopts the photoinduced charge transfer mechanism and the conjugate passivation mechanism, therefore, a response range respect to hydrazine can be expanded; the probe has the characteristics of being fast in response, high in sensitivity and high in selectivity, is suitable for being applied to safety detection of foods as well as safety detection of a laboratory, in particular applied to industrial wastewater monitor; and the probe has a wide application prospect in environment monitoring, ecological protection, disease diagnosis, industrial production and pollution discharge inspection.
Owner:浙江富昇科技有限公司
Synthetic method of malononitrile
InactiveCN103044286AHigh purityImprove stabilityPreparation by carboxylic acid amide dehydrationPtru catalystDistillation
The invention discloses a synthetic method of malononitrile. Cyanoacetamide reacts with triphosgene in the presence of a catalyst to synthesize malononitrile, wherein the catalyst is any one substance or a mixture of multiple substances selected from N,N-dimethyl formamide, sodium chloride, pyridine and triethylamine. The method provided by the invention employs the triphosgene as a dehydrating agent, and the triphosgene is low in cost, available and high in stability, and facilitates storage and transportation; the reaction products of the cyanoacetamide and the triphosgene only include carbon dioxide and hydrogen chloride gases except for the malononitrile, without solid waste, and therefore, after the reaction is completed, the steps of removing the solid waste by means of filtering, centrifuging and the like are not needed; the reaction mixture is directly subjected to reduced pressure distillation after the solvent is recovered so that the malononitrile having the purity of higher than 98% can be obtained; and the posttreatment is simple.
Owner:CHONGQING UNISPLENDOUR CHEM
Quinoline nitrile derivative with aggregation-induced emission performance
ActiveCN102702096AStrong fluorescenceSignificant aggregation-induced luminescence characteristicsOrganic chemistryLuminescent compositionsQuinolineOrganism
The invention relates to a quinoline nitrile derivative. A method comprises the following steps of: reacting 2-methylquinoline serving as an initial raw material with the corresponding alkyl halide to obtain quaternary ammonium salt, performing Michael addition-elimination reaction on the quaternary ammonium salt and malononitrile, and performing Knoevenagel condensation reaction on the obtained compound and the corresponding aromatic aldehyde to obtain a target product. The aggregate or solid substance of the derivative has strong fluorescence and large wavelength, is a good aggregation-induced emission material, and has considerable application prospects in the fields of electroluminescent devices, fluorescent probes, intelligent materials, organism imaging and the like.
Owner:EAST CHINA UNIV OF SCI & TECH
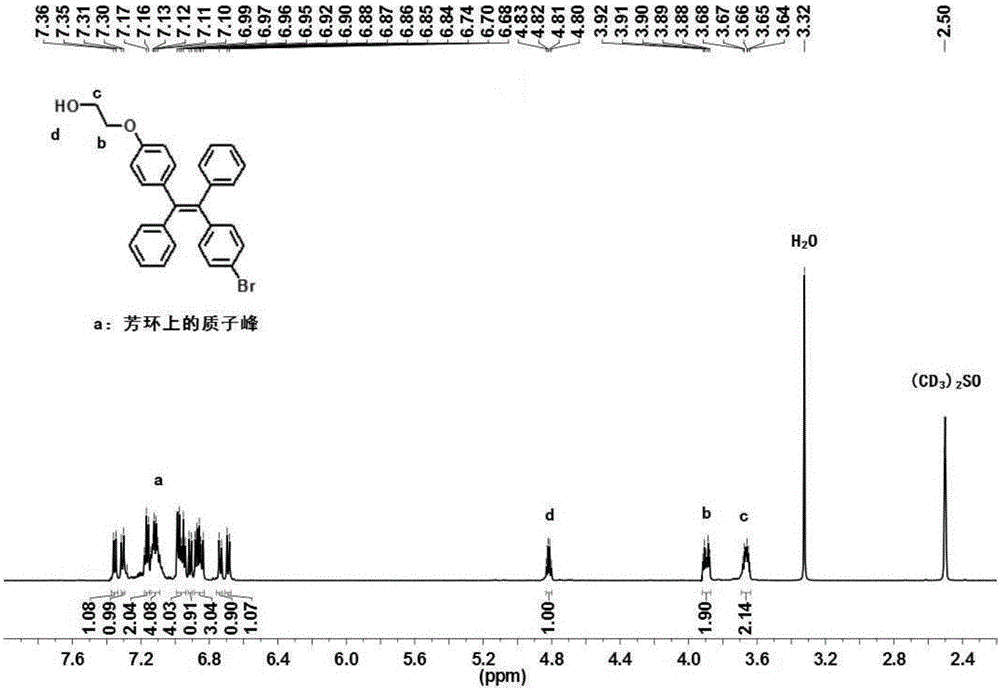
Malononitrile-substituted aryl anthracene-phenanthrene organic electroluminescent material and preparation method and application thereof
ActiveCN108586289AHas thermally activated delayed fluorescence propertiesImprove performanceOrganic chemistrySolid-state devicesFluorescenceTriplet state
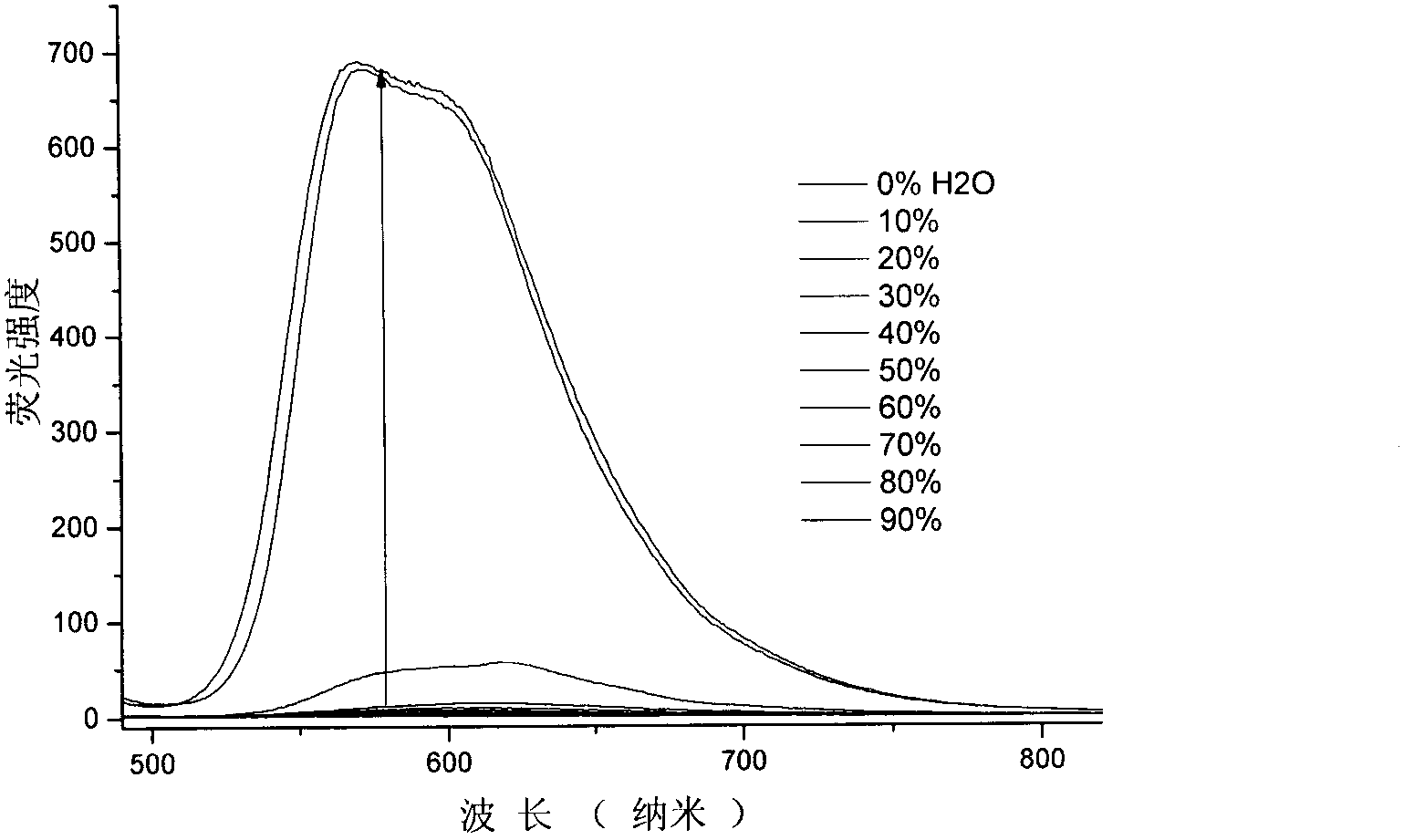
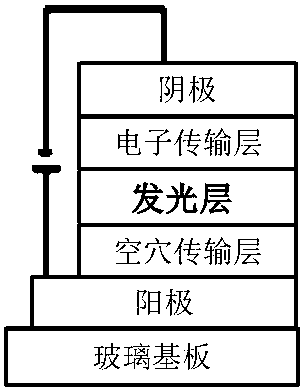
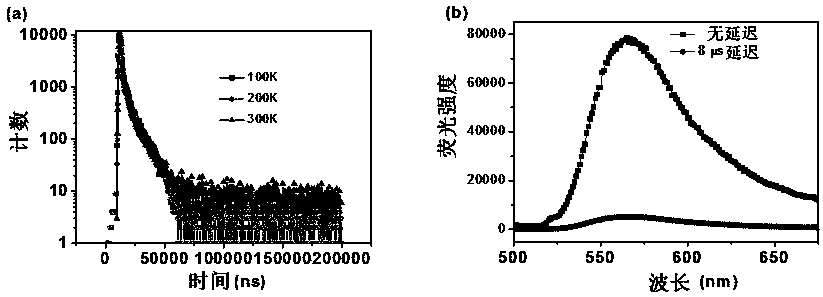
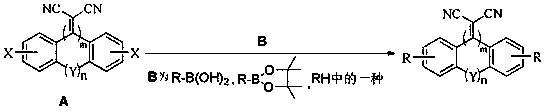
The invention discloses a malononitrile-substituted aryl anthracene-phenanthrene organic electroluminescent material and a preparation method and application thereof. The organic electroluminescent material uses malononitrile-substituted aryl anthracene-phenanthrene as a parent nucleus and a structural formula of the organic electroluminescent material is as shown in a formula (I). The malononitrile-substituted aryl anthracene-phenanthrene organic electroluminescent material provided by the invention is simple in preparation process; HOMO and LUMO energy level electron cloud can be effectivelyseparated; a singlet state and triplet state energy gap (delta EST) is small; triplet-state excitonic luminescence can be converted into singlet-state excitonic luminescence by RIST (Reverse InterSystem Traversing); the malononitrile-substituted aryl anthracene-phenanthrene organic electroluminescent material has the thermal activation delayed fluorescence property; when the malononitrile-substituted aryl anthracene-phenanthrene light-emitting material provided by the invention is applied to an OLED (Organic Light-Emitting Diode) light-emitting device, high device efficiency is obtained. (theformula (I) is shown in the specification).
Owner:NORTHWEST UNIV
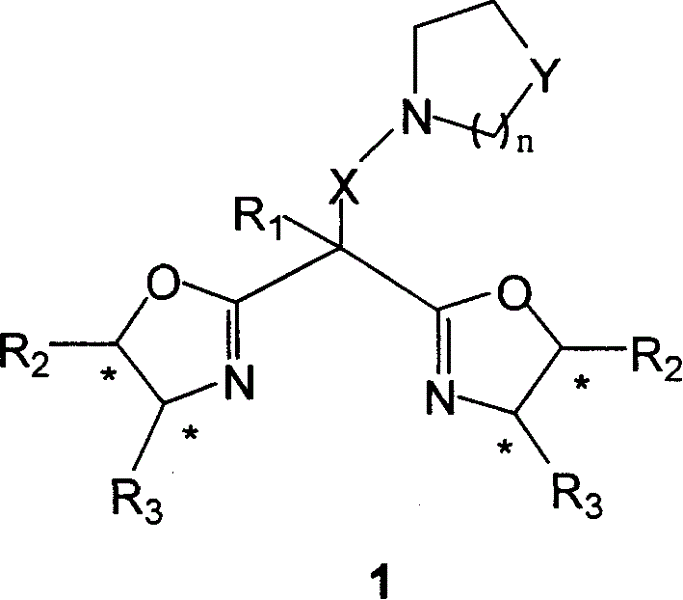
Dual functions ligand compound of chirality dioxazoline, preparation and application
InactiveCN1626524AEasy to getEasy to manufactureOrganic chemistryOrganic-compounds/hydrides/coordination-complexes catalystsChemical compoundSubstitution reaction
A bifunctional chiral bioxazoline ligand used as the catalyst for asymmetrical reactions, such as cyanosiliconizing reaction, is prepared from propylbinitrile or its derivative through substitution reaction and cyclizing reaction on chiral aminoalcohol, or from the bioxazoline methane through substitution reaction.
Owner:PEKING UNIV
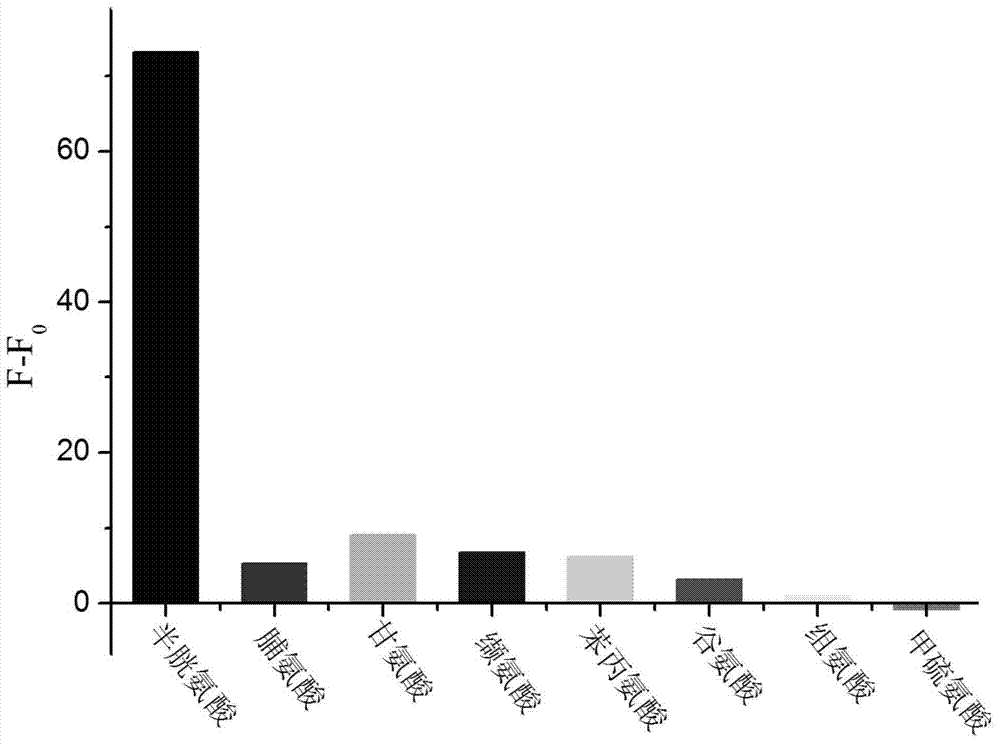
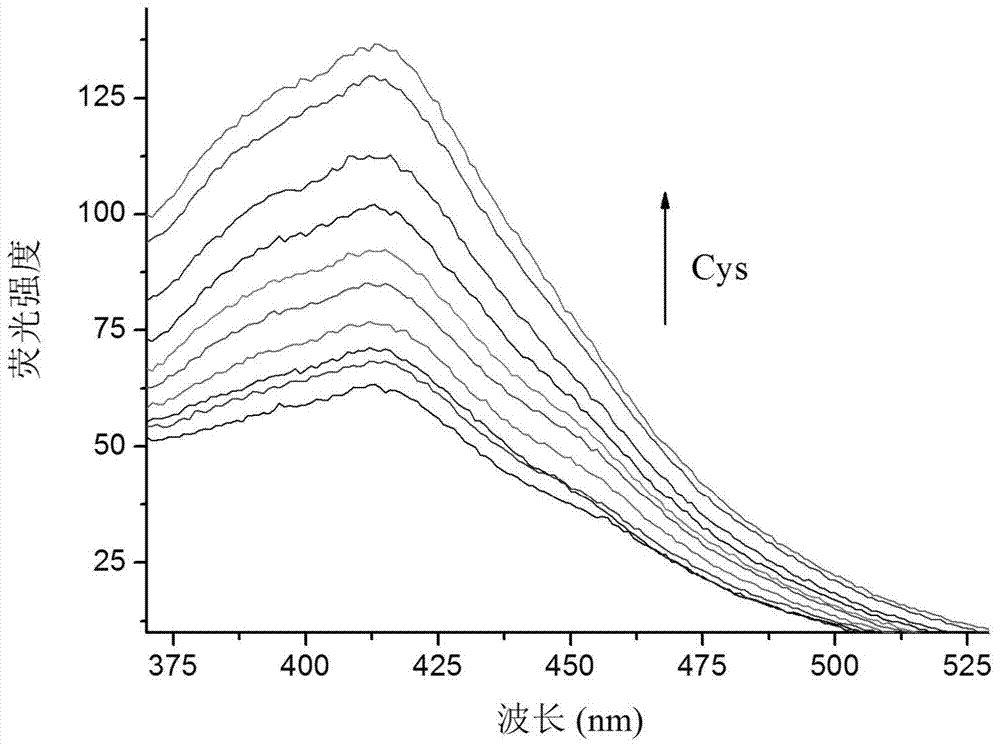
Coumarin derivatives as well as preparation method and application thereof
InactiveCN103570701ANo significant fluorescence responseHigh fluorescence intensityOrganic chemistryBiological testingDiseaseFluorescence
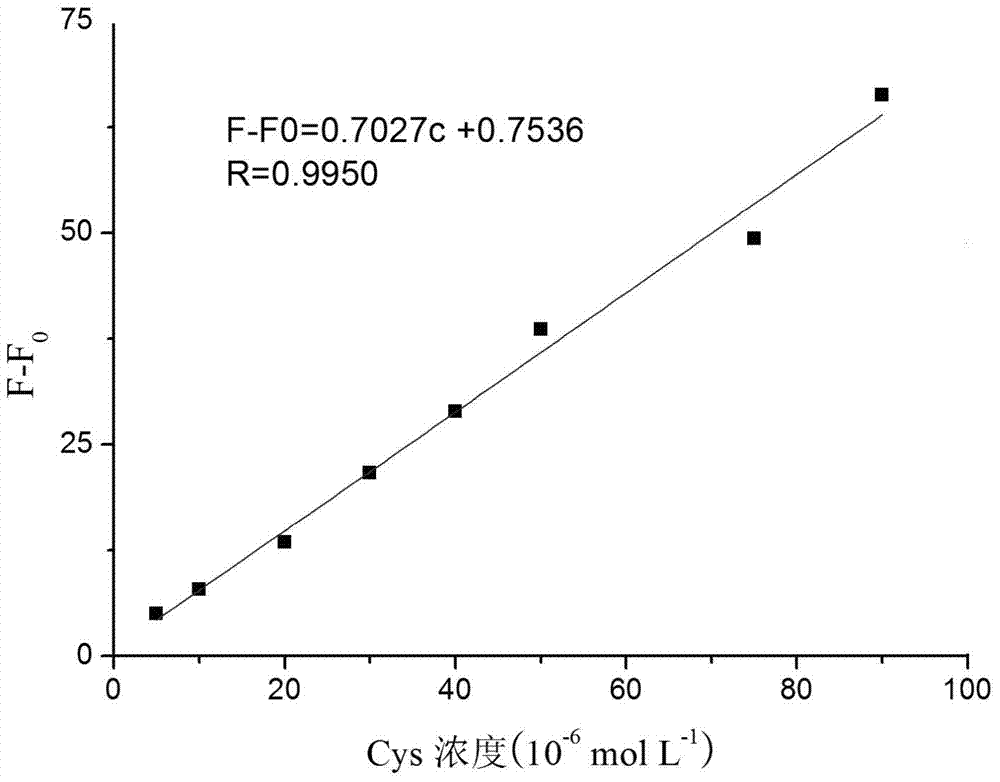
The invention provides coumarin derivatives as well as a preparation method and application thereof. The coumarin derivatives have the molecular formula: Cl3H6N2O4. The preparation method comprises the steps: firstly, preparing 4-chloromethyl-7-hydroxycoumarin, 4-hydroxymethyl-7-hydroxycoumarin and 7-hydroxycoumarin-4-formaldehyde; and then, adding the 7-hydroxycoumarin-4-formaldehyde into water, next, adding malononitrile and 1-methylimidazole, stirring at the room temperature, and filtering to obtain 2, 2-dicyano-3-(7-hydroxyl-4-coumarinyl)oxacyclopropane. The 2, 2-dicyano-3-(7-hydroxyl-4-coumarinyl)oxacyclopropane prepared by the preparation method can be used as a fluorescent probe for selectively detecting cysteine in an organism and can also be used for fluorescently marking cysteine in a cell so as to provide help for the diagnosis of related diseases in clinical medicals.
Owner:SHANXI UNIV
Method for preparing (Z)-5-amino-alpha-(ethoxy imino group)-1, 2, 4-thiadiazole-3-acetic acid
ActiveCN103804321AShort synthetic routeFew synthetic stepsOrganic chemistryAcetic acidPotassium thiocyanate
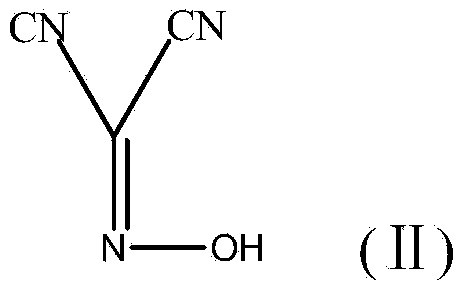
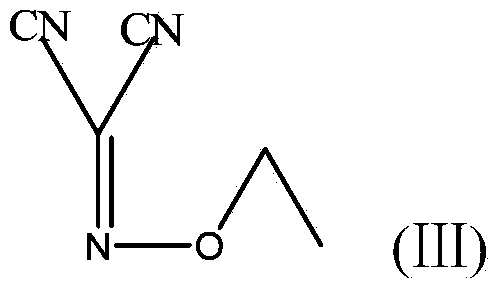
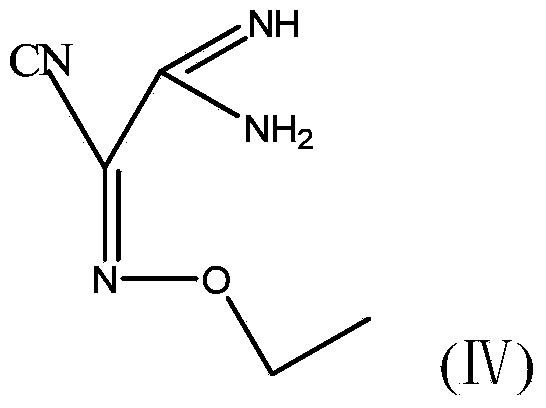
The invention discloses a method for preparing (Z)-5-amino-alpha-(ethoxy imino group)-1, 2, 4-thiadiazole-3-acetic acid. The method comprises steps of firstly, carrying out oximation reaction on malononitrile and sodium nitrite under the action of acetic acid so as to obtain a compound A, then carrying out Williamson synthesis on the compound A and bromoethane or diethyl sulfate under alkaline condition, so as to obtain a compound B, then carrying out amidine reaction on the compound B and ammonia water, so as to obtain a compound C, carrying out cyclization reaction on the compound C and potassium rhodanide for ring closing so as to obtain a compound D, and finally, hydrolyzing the compound D under the action of a strong base, so as to obtain (Z)-5-amino-alpha-(ethoxy imino group)-1, 2, 4-thiadiazole-3-acetic acid. The method has few synthesis steps, has low cost, uses the materials which are cheap and easy to obtain, is beneficial to industrial production, and has light contamination; the purity of the product can reach 99%, so that synthesis of high-purity ceftaroline fosamil in the subsequent step is guaranteed.
Owner:山西海泰电子材料有限公司
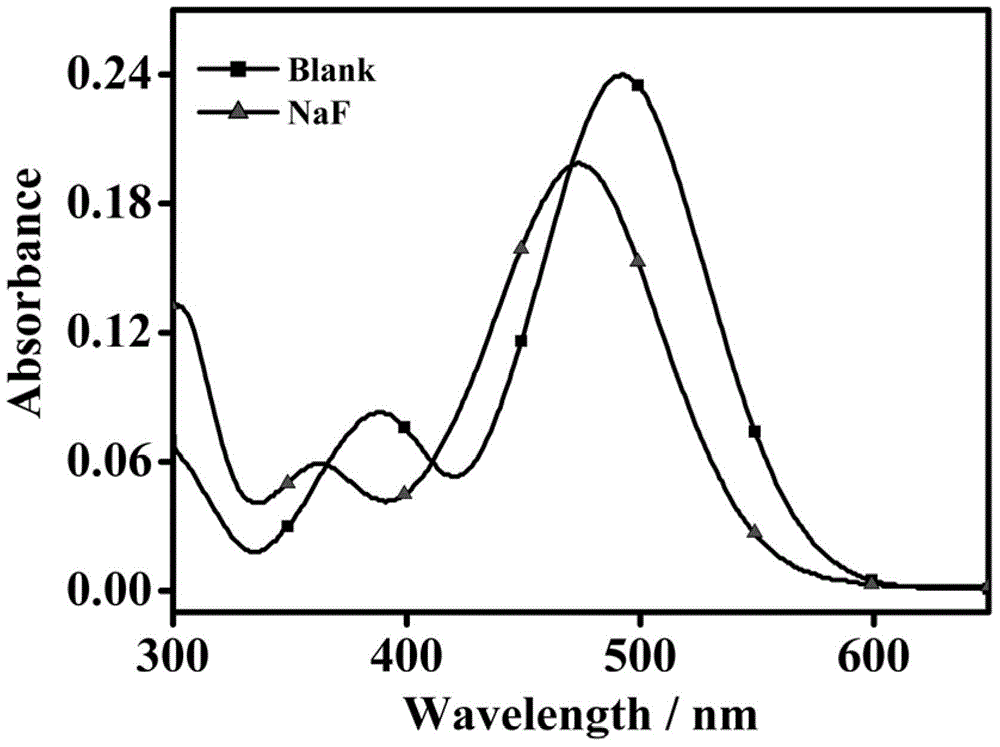
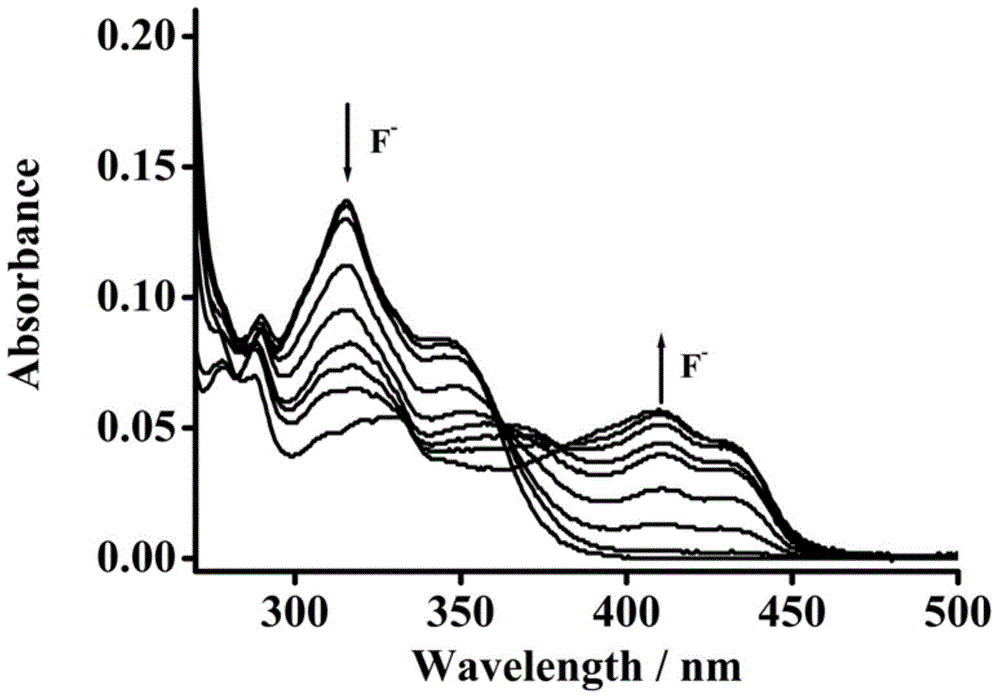
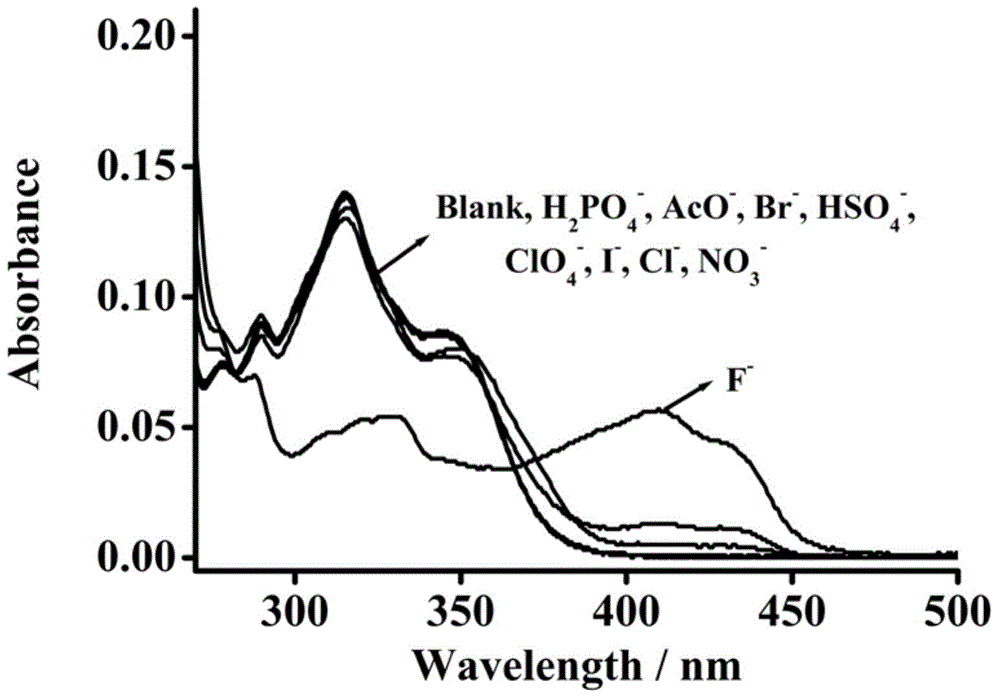
Fluorescent molecular probe for detecting fluoride ions in aqueous solutions as well as synthesis method and application thereof
ActiveCN104418874AEasy to synthesizeMild reaction conditionsGroup 4/14 element organic compoundsFluorescence/phosphorescenceHydrogen SulfateSulfate radicals
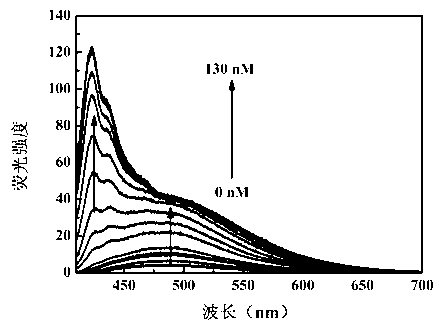
The invention relates to a preparation method of a fluorescent molecular probe for detecting fluoride ions in aqueous solutions through fluorescence enhancement and an application of the fluorescent molecular probe to detecting fluoride ions. The fluorescent molecular probe is prepared by protecting 1,4-diethyl-1,2,3,4-tetrahydro-7-hydroxyquinoxaline-6-aldehyde taken as a raw material with silane and then condensing the raw material and malononitrile. The fluorescent molecular probe is simple and convenient to synthesize, and reaction conditions are mild. The fluorescent molecular probe has the specific characteristics that the probe molecule has stable optical properties and higher synthetic yield; the probe molecule has high sensitivity of detection of fluoride ions in the aqueous solutions and low lower limit of detection, and the limit of detection is 5.4mu M; the response range is 0-1mM and the detection range is wide; the probe molecule has good selectivity and has no responses to anions, such as chloride ions, bromide ions, iodide ions, tetrabutylammonium cyanide, nitrates radicals, hydrosulfate radicals, perchlorate radicals, acetate radicals, thiocyanate radicals, azide radicals, cysteine, bovine serum albumin, carbonate radicals, sulfate radicals and reduced glutathione; the fluorescent molecular probe has practical application values in the fields of biochemistry, environmental sciences and the like.
Owner:SUZHOU ROWLAND BIOTECH
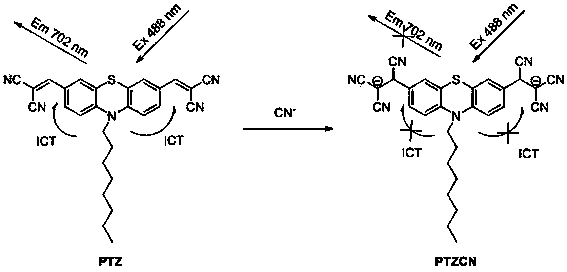
Fluorescent probe as well as preparation method and application thereof
InactiveCN104357044AEasy to synthesizeFew synthetic stepsOrganic chemistryFluorescence/phosphorescenceFluoProbesFluorescence
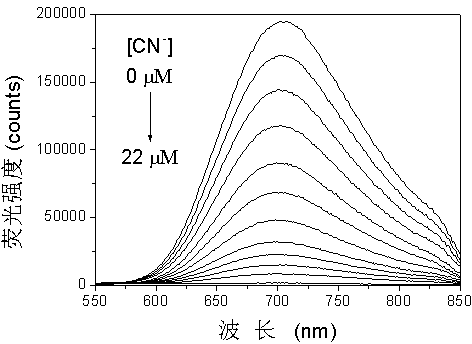
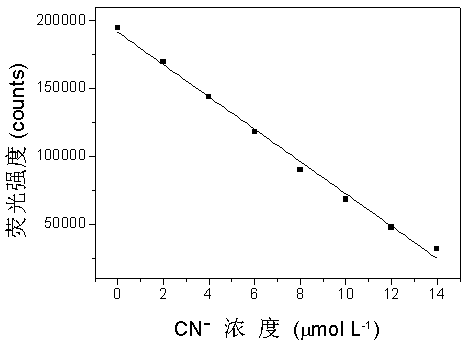
The invention provides a fluorescent probe. The structural formula of the fluorescent probe is represented by general formula I shown in the specification, wherein R is one of C7-C12 alkyl groups. The invention further provides a preparation method of the fluorescent probe and an application of the fluorescent probe to detection of cyanide ions. A phenothiazine derivative is used as a fluorescent parent, a malononitrile structure is introduced as an active center reacting with the cyanide ions, and selective detection of the cyanide ions is realized by the aid of fluorescent differences between reactants and products. The prepared fluorescent probe PTZ is excited at 488 nm and emitted at 702 nm, has very high selectivity and sensitivity for the cyanide ions and is high in response speed, the lowest concentration of the cyanide ions is detected to be 67 nmol / L<-1>, the cyanide ions can be detected quantitatively, and the fluorescent probe can be applied to detection of the cyanide ions in water bodies, soil and organisms.
Owner:SHANGHAI UNIVERSITY OF ELECTRIC POWER
Application of high selectivity reaction type fluorescence probe for detecting hydrogen sulfide
InactiveCN107216270ASensitive detectionThe synthesis process is simpleOrganic chemistryFluorescence/phosphorescenceFluorescenceFine chemical
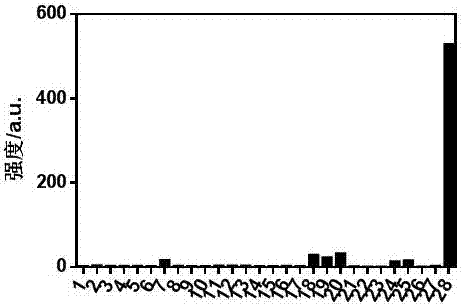
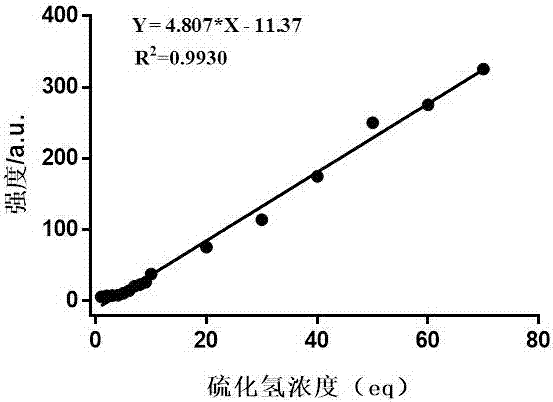
The invention relates to an application of a highly selective reactive fluorescent probe for detecting hydrogen sulfide, which belongs to the field of fine chemical industry. The specific probe is 2‑{(6‑(dimethylamino)‑3‑(2,4‑dinitrophenoxy)naphthylacetamide‑2‑)methylene}malononitrile, which can be used to detect The presence of hydrogen sulfide in the in vitro environment and living cells is quantitatively determined within a certain range. The specific determination process of hydrogen sulfide is as follows: select 2‑{(6‑(dimethylamino)‑3‑(2,4‑dinitrophenoxy)naphthylacetamide‑2‑)methylene}malononitrile for thiolysis The buckle reaction is a probe reaction, and the hydrogen sulfide content in each system is determined by quantitatively detecting the amount of reaction products produced by selecting an appropriate probe concentration within the linear reaction range.
Owner:南京微瑞莱电子科技有限公司
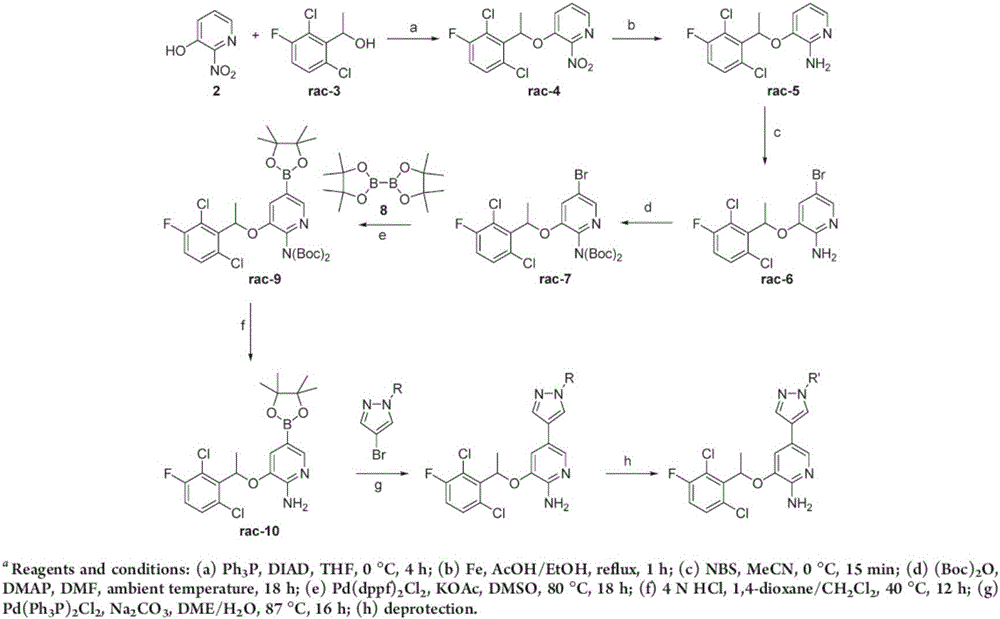
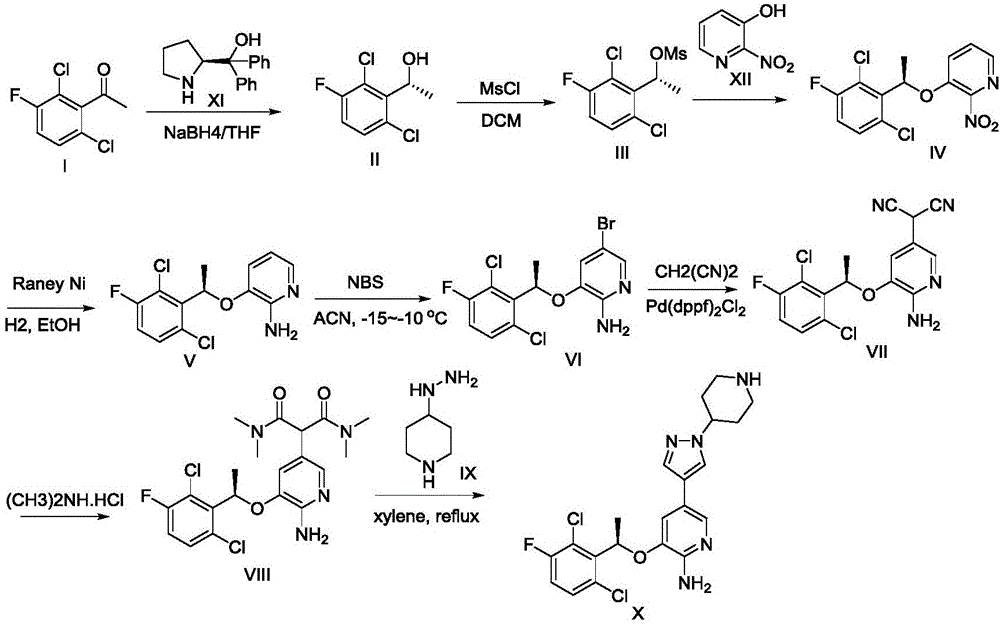
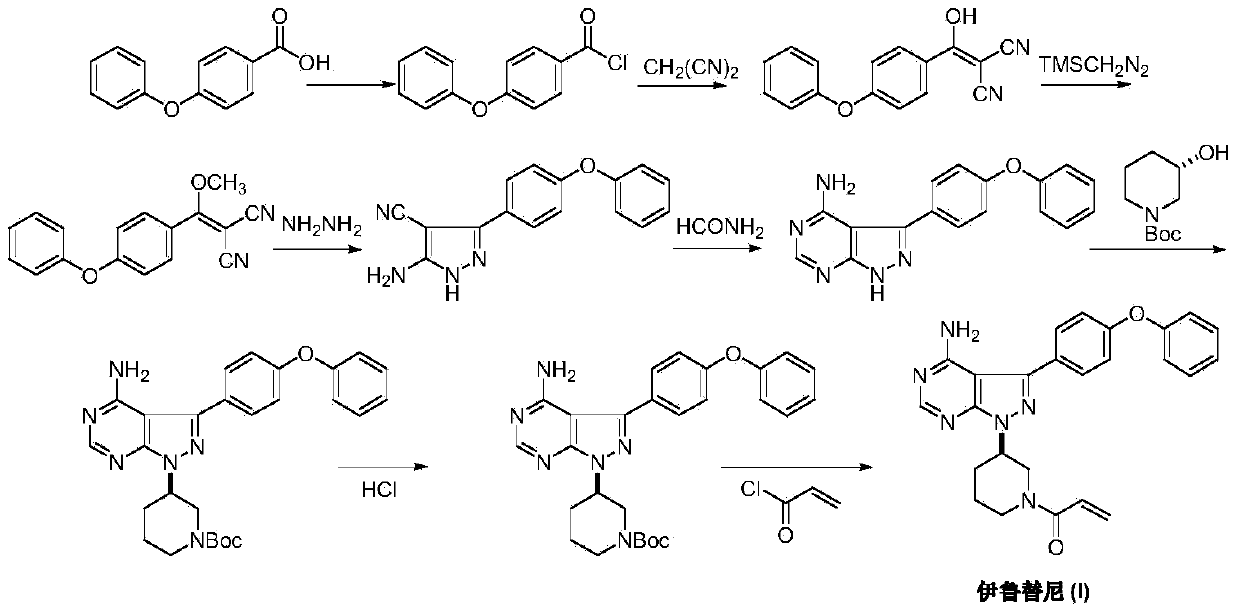
Method for synthesizing intermediate 4-amino-3-(4-phenoxy-phenyl)-1H-pyrazolo[3,4-d]pyrimidine of Ibrutinib
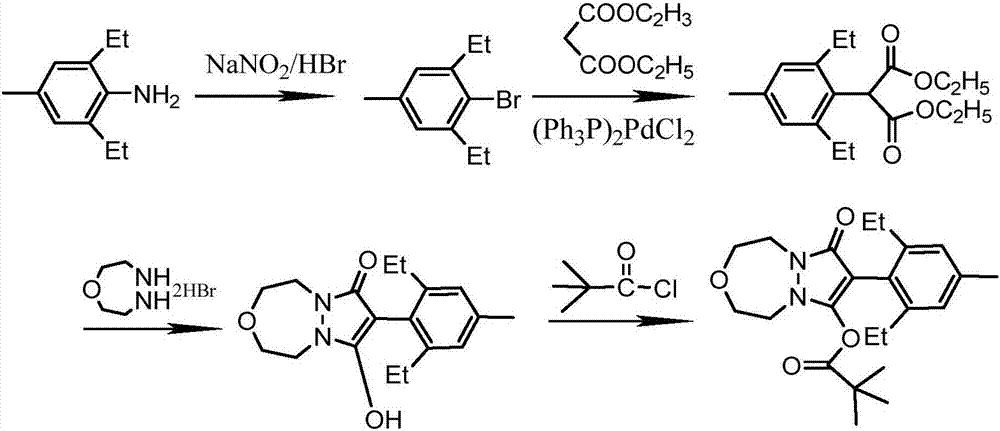
The invention discloses a method for synthesizing an important intermediate 4-amino-3-(4-phenoxy-phenyl)-1H-pyrazolo[3,4-d]pyrimidine of Ibrutinib. The method comprises the following steps: step one, condensing malononitrile and triethyl orthoformate and then carrying out a one-pot reaction with hydrazine hydrate to obtain 5-amino-4-cyano-pyrazole; step two, condensing the 5-amino-4-cyano-pyrazole with formamide to prepare a compound of formula (II) 4-amino-1H-pyrazolo[3,4-d]pyrimidine; step three, carrying out a bromination reaction of the compound of formula (II) and brominating agent to obtain a compound of formula (III) 4-amino-3-bromine-1H-pyrazolo[3,4-d]pyrimidine; step four, carrying out a Stille reaction on the compound of formula (III) and trimethyl p-phenoxy phenyltin under the catalyst effect of metal palladium to prepare a compound of formula (I) 4-amino-3-(4-phenoxy-phenyl)-1H-pyrazolo[3,4-d]pyrimidine. According to the method for synthesizing the important intermediate 4-amino-3-(4-phenoxy-phenyl)-1H-pyrazolo[3,4-d]pyrimidine of Ibrutinib provided by the invention, raw materials are low in price and easily obtained, the reaction conditions are moderate, the operation is simple and convenient, the method is suitable for industrial production, and a new way is provided for preparing Ibrutinib and intermediate.
Owner:HUAIHAI INST OF TECH
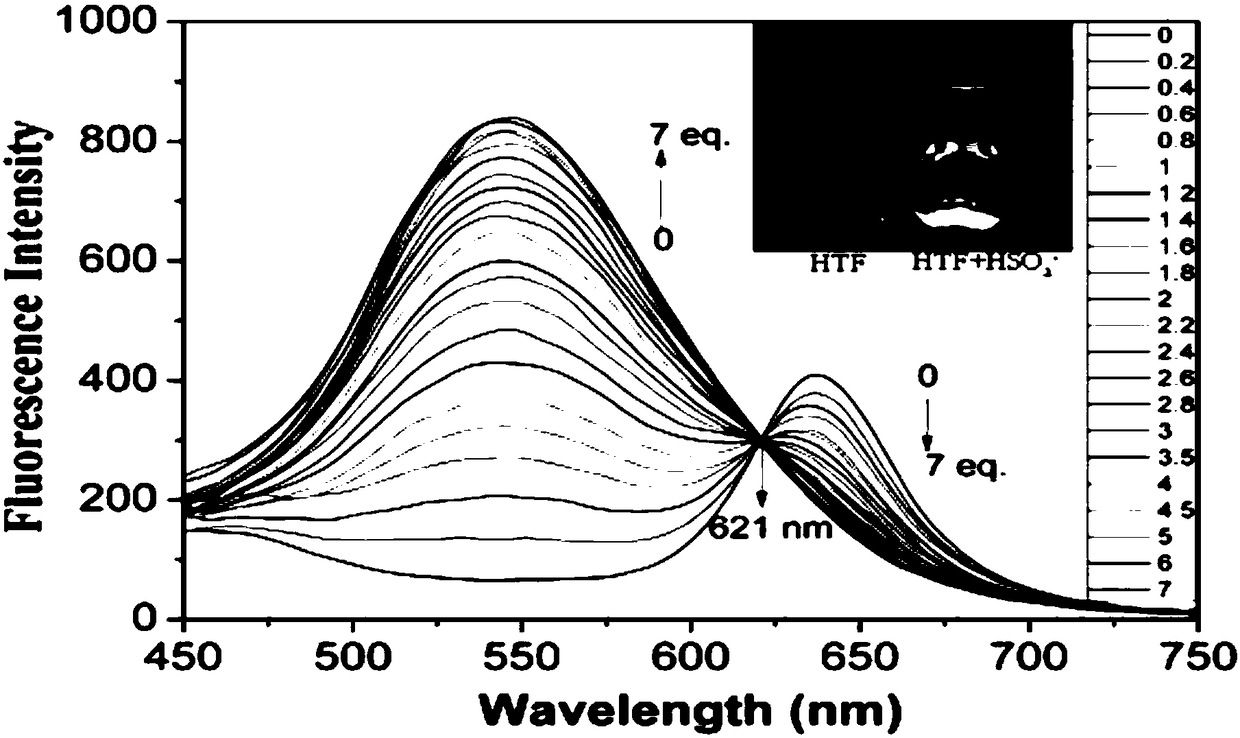
Ratiometric fluorescent probe for detecting bisulfite and application of ratiometric fluorescent probe
ActiveCN108129428AFluorescence ratio response effectEnable ratiometric imagingOrganic chemistryFluorescence/phosphorescenceEnergy transferResonance
The invention discloses a ratiometric fluorescent probe for detecting bisulfite, which is obtained by taking dansulfonyl fluorophore as a donor, taking (E)-2-(3-cyano-4-(4-(dimethylamino) styrene)-5,5-dimethylfuran-2(5H)-subunit)malononitrile fluorophore as a receptor, using piperazine linking and basing on a fluorescence resonance energy transfer mechanism. The invention also discloses application of the ratiometric fluorescent probe in detection of a sample containing the bisulfite and positioning imaging of intracellular lipid droplets. The ratiometric fluorescent probe provides a powerfultool for correlated detection or targeting localization in the field of biomedicine, is expected to play a role in biological sciences research and has a broad application prospect.
Owner:SHANDONG UNIV
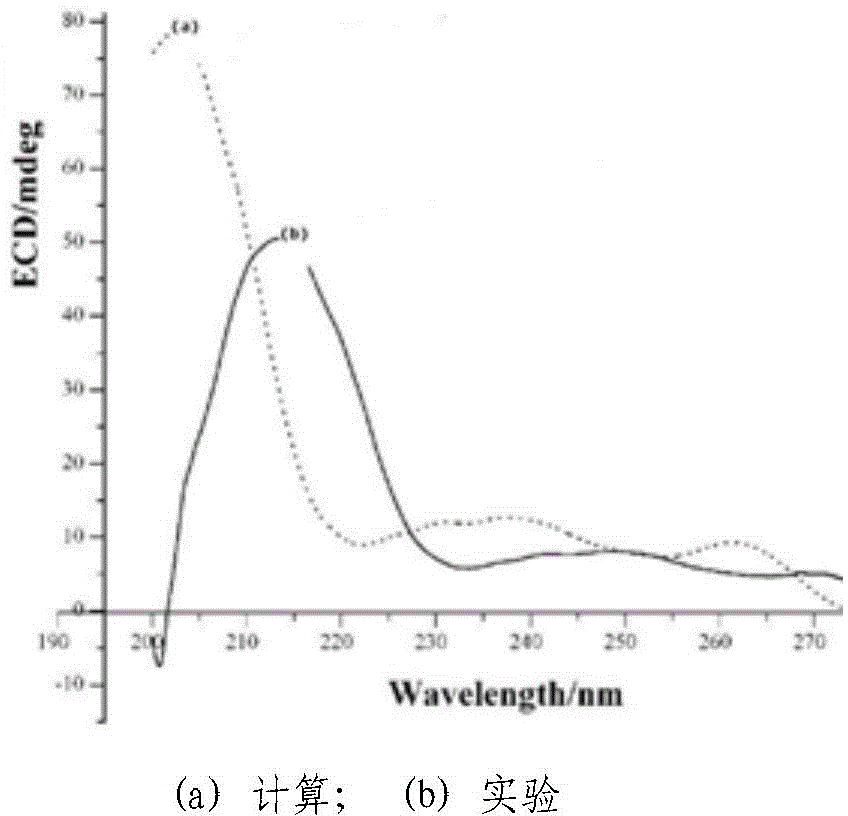
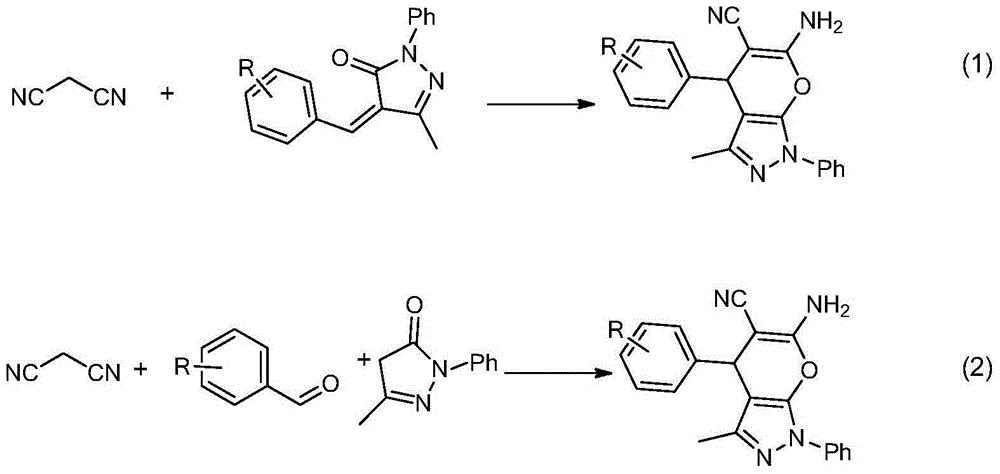
Chiral 1, 4-dihydropyran (2, 3-c) pyrazole derivative as well as synthesis method and application of chiral 1, 4-dihydropyran (2, 3-c) pyrazole derivative
ActiveCN103910737AFlexible response timeHigh yieldOrganic active ingredientsOrganic chemistrySynthesis methodsSolvent
The invention discloses a chiral 1, 4-dihydropyran (2, 3-c) pyrazole derivative as well as a synthesis method and application of the chiral 1, 4-dihydropyran (2, 3-c) pyrazole derivative. The synthesis method comprises the steps of with methylbenzene as a solvent, enabling a substituted or unsubstituted imidazolone derivative and propanedinitrile to react under the analysis of a Takemoto catalyst, and carrying out after-treatment on a reaction product to obtain the chiral 1, 4-dihydropyran (2, 3-c) pyrazole derivative. The method has the advantages of flexible reaction time, relatively high yield, simplicity and convenience in operation and the like, and is wide in application range. The chiral 1, 4-dihydropyran (2, 3-c) pyrazole derivative has an inhibiting effect on gram positive bacteria and gram negative bacteria.
Owner:惠州拓康生物科技有限公司
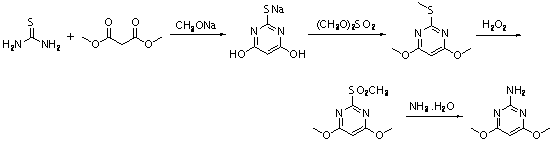
Method for preparing 2-amino-4, 6-dimethoxypyrimidine
ActiveCN103159684ARaw materials are cheap and easy to getEasy to operateOrganic chemistryEconomic benefitsSolvent
The present invention relates to a method for preparing 2-amino-4, 6-dimethoxypyrimidine (ADMP). According to the method, malononitrile is uses as a raw material, special solvents are adopted, and in the action of a specific catalyst, after reactions of imidization, cyanamide substitution, aromatic cyclization, etc., 2-amino-4, 6-dimethoxypyrimidine of high purity is synthesized in a high yield. The method has no harsh conditions in all the steps, is simple in operation, little in the amounts of waste water, waste gas and industrial residues, and environmentally friendly, thereby having significant social and economic benefits, and being suitable for industrial production.
Owner:HUBEI HUIDA HIGH TECH CO LTD
Green catalytic preparation method of pyrano[4,3-b]pyran derivative
ActiveCN105037381AIncrease acidityHigh catalytic activityOrganic chemistryFiltrationReaction temperature
The invention discloses a green catalytic preparation method of a pyrano[4,3-b]pyran derivative and belongs to the technical field of ionic liquid catalysis. In the preparation reaction, the molar ratio of aromatic aldehyde, 4-hydroxy-6-methyl-2-pyrone and malononitrile is 1:1:1, the molar weight of a bissulfonate ionic liquid catalyst is 3-5% of that of aromatic aldehyde, the volume dose of reaction solvent water by milliliter is 5-8 times of the molar weight of aromatic aldehyde by millimole, the reaction temperature is 60-80 DEG C, the reaction time is 15-40 min, cooling to the room temperature is performed after finish of reaction, lots of solids are separated out and crushed, then leaving to stand and suction filtration are carried out, and the obtained filter residues are recrystallized by ethanol after being dried to obtain the target product. Compared with preparation methods adopting other acidic ionic liquid catalysts, the green catalytic preparation method has the characteristics that the catalytic activity of the catalyst, the biodegradability and the raw material utilization ratio are high, and the whole preparation process is simple and convenient to operate, and industrial large-scale application is facilitated.
Owner:平邑现代中药产业园有限公司
Fluorescent molecular probe for detecting fluoride ions as well as synthesis method and application thereof
InactiveCN104418875AHigh sensitivityStable fluorescenceGroup 4/14 element organic compoundsMaterial analysis by observing effect on chemical indicatorHigh fluoridePhosphate
The invention relates to a preparation method of a fluorescent molecular probe for detecting fluoride ions through colorimetric detection and fluorescence enhancement and an application of the fluorescent molecular probe to detecting fluoride ions. The fluorescent molecular probe is prepared by protecting 2-hydroxyl-1-naphthaldehyde taken as a raw material with silane and then condensing the raw material and malononitrile. The fluorescent molecular probe is simple and convenient to synthesize, and reaction conditions are mild. The fluorescent molecular probe has the specific characteristics that the probe molecule has stable optical properties and higher synthetic yield; the probe molecule has high fluoride ion detection sensitivity and low lower limit of detection, and the limit of detection is 0.52mu M; the response range is 0-100mu M and the detection range is wide; the probe molecule has good selectivity and has no responses to anions, such as dihydrogen phosphate radicals, acetate radicals, bromide ions, hydrosulfate radicals, chlorate radicals, iodide ions, chloride ions and nitrate radicals; the probe molecule is suitable for colorimetric detection; the fluorescent molecular probe has practical application values in the fields of biochemistry, environmental sciences and the like.
Owner:SUZHOU ROWLAND BIOTECH
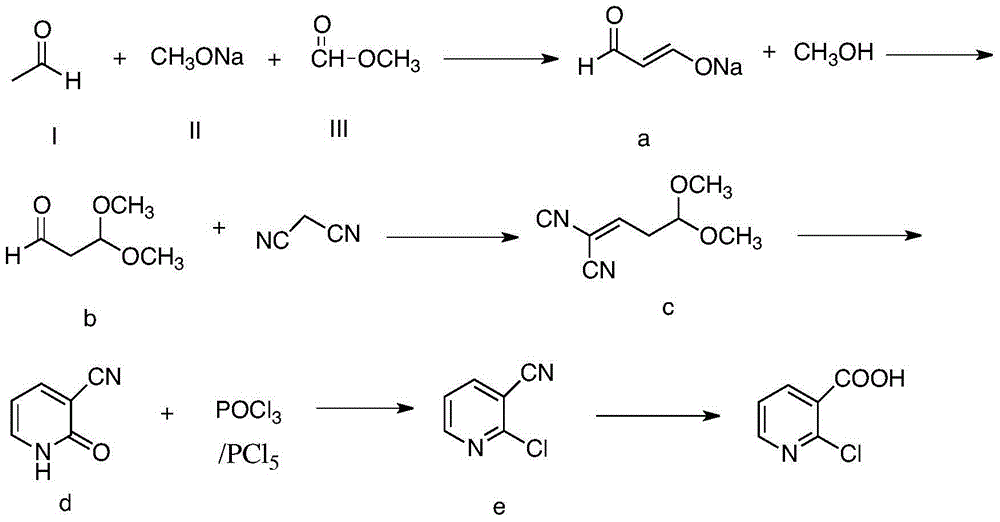
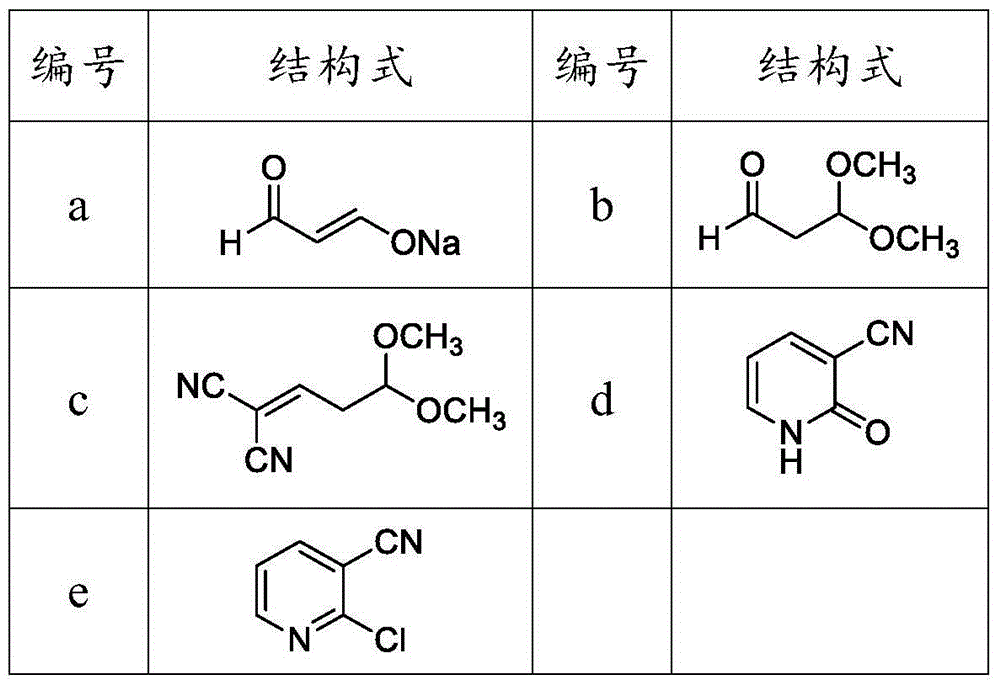
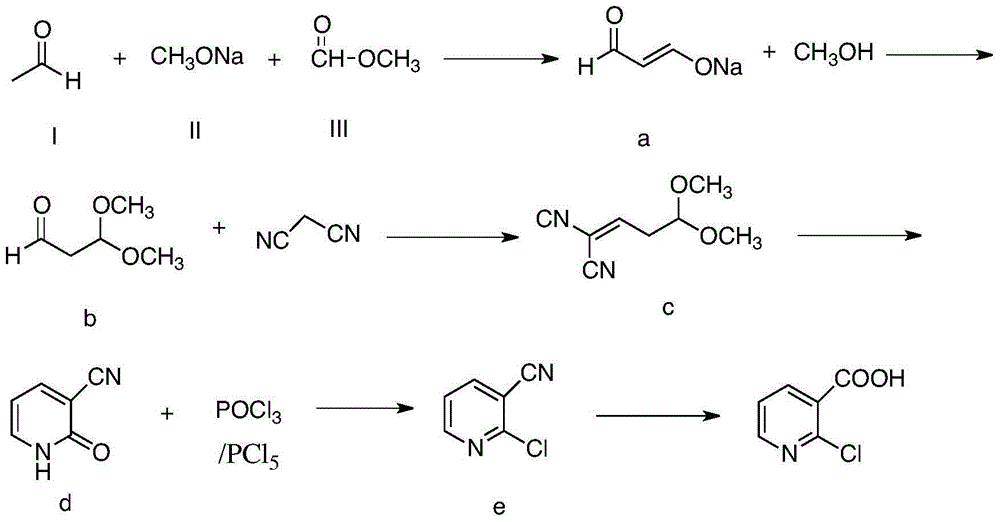
Method for preparing 2-chloronicotinic acid
The invention discloses a green and environment-friendly method for preparing 2-chloronicotinic acid. The implementation process of the method comprises the following steps: generating aldehyde sodium ethenolate by taking acetaldehyde, sodium methylate and methyl formate as raw materials; reacting with methyl alcohol to generate 1,1-dimethoxy propionaldehyde; reacting with malononitrile to generate a cyclization precursor; and carrying out cyclization, chlorination and cyan-hydrolysis to generate the 2-chloronicotinic acid. The method provided by the invention is novel in line, mild in reaction condition of each step, relatively high in yield, less in generated wastewater and beneficial for reducing the production cost of the 2-chloronicotinic acid, and has industrialization condition. By selecting industrialization products such as the acetaldehyde, the sodium methylate and the methyl formate as raw materials which are easy to get, low in cost, mild and efficient in reaction condition, green and environment-friendly, the invention provides a new way which is novel and efficient and can be used for industrially producing the 2-chloronicotinic acid.
Owner:JIANGSU ZHONGBANG PHARMA
Organic conjugated molecule capable of being processed by solution and application thereof in solar cells
InactiveCN101787020AThe synthesis method is simpleEasy to purifyOrganic chemistryFinal product manufactureHeterojunctionPyran
The invention, which belongs to the technical field of solar cells, relates to a donor-acceptor type organic conjugated molecule based on 2-pyran-4-ylidene malononitrile as acceptor and the application of the organic conjugated molecule in bulk heterojunction solar cells. The invention provides a series of low-HOMO energy level (high open circuit voltage and high stability), narrow-bandgap, high-absorbability and high-mobility donor-acceptor type organic conjugated molecule solar cell materials by selecting different donor units to couple with 2-pyran-4-ylidene malononitrile (acceptor unit); when the organic conjugated molecule is used in the production of solar cells, an element with a high open circuit voltage and high photoelectric conversion efficiency can be obtained; and moreover, the element production technique is simple, can be easily repeated, and is favorable for the industrialized batch production of elements.
Owner:JILIN UNIV
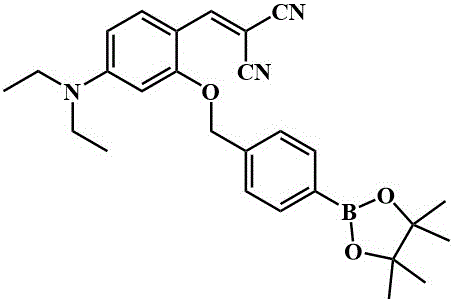
Fluorescent compound and application thereof in hypochlorous acid detection
InactiveCN106045950AEasy to launchPromote specific responseOrganic chemistryFluorescence/phosphorescenceFluorescenceChemical compound
The invention belongs to the technical field of analysis and detection and discloses a fluorescent compound and the application thereof in hypochlorous acid detection. The fluorescent compound is 2-{3-cyan-4-[2-(4'-{2-[4-(2-hydroxy-ethyoxyl)-phenyl]-1,2-biphenyl-vinyl}-biphenyl-4-yl)-vinyl]-5,5-dimethyl-5H-furyl-2-ylidene}-malononitrile, and has a structural formula as shown in formula (1). By the adoption of the fluorescent compound, enhancement mode fluorescence detection of hypochlorous acid can be achieved, interference of external conditions can be avoided during analysis and detection, and detection precision and accuracy are high; the fluorescent compound can be prepared into test paper which is convenient to carry and store and capable of achieving qualitative testing conveniently, and fluorescence quenching occurring when traditional fluorochrome is used as a solid material is avoided. The fluorescent compound can be used for qualitative and quantitative analysis of hypochlorous acid in chemical and environmental samples as well as other samples.
Owner:SOUTH CHINA UNIV OF TECH
Preparation and application of hydrogen peroxide fluorescent probe compound
InactiveCN106220663AInnovative designGood choiceGroup 3/13 element organic compoundsFluorescence/phosphorescenceFluorescenceNitrogen gas
The invention relates to a reactive type hydrogen peroxide fluorescent probe compound and preparation and application thereof. The hydrogen peroxide fluorescent probe compound has the structure as shown in a formula I. The preparation method comprises the steps that 4-diethylaminosalicylaldehyde and 4-bromobenzyl bromide are dissolved in acetone, anhydrous potassium carbonate is taken as a catalyst, a refluxing reaction is conducted under the protection of nitrogen, and a product II is obtained; the product II and bis(pinacolato)diboron react under the protection of nitrogen by taking [1,1.-bis(diphenylphosphino)ferrocene]dichloropalladium as a catalyst, and a product III is obtained; the product III and malononitrile are subjected to stirring at room temperature by taking piperidine as a catalyst, and the final product probe I is obtained. The probe compound has good selectivity and sensitivity to hydrogen peroxide, is low in detection limit and free of toxicity to cells and can be applied to detection and imaging of hydrogen peroxide in the cells.
Owner:UNIV OF JINAN
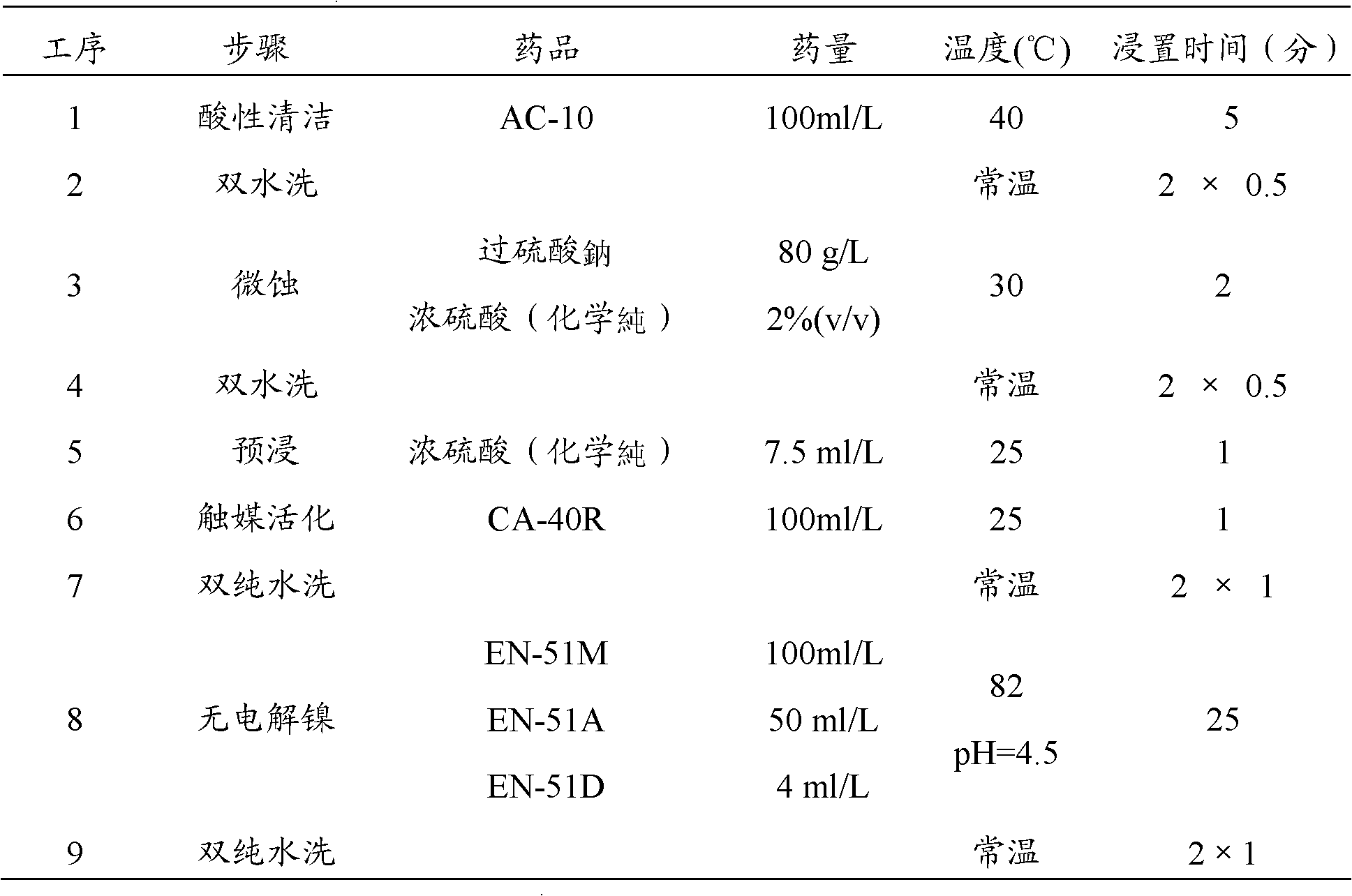
A displacement chemical gold plating solution
InactiveCN102286736AExcellent adhesionAvoid corrosionLiquid/solution decomposition chemical coatingPotassiumDiethylenetriamine
The invention discloses a displacement type electroless gold plating solution, which comprises monopotassium citrate monohydrate bis(malononitrile alloy (I)), a conductive compound, a buffering agent, a shielding complexing agent, a nickel oxidation remover and water. The nickel oxidation remover is one or more of diethylenetriamine, pentamethyldiethylenetriamine, triethylenetetramine, hexamethyltriethylenetetramine and tetraethylenepentamine. The present invention provides the source of gold ions with potassium citrate monohydrate (malononitrile alloy (I)) as the displacement type chemical gold plating solution, thus does not need to use potassium gold cyanide as the gold source compound, and is an environmentally friendly displacement chemical Gold plating solution. Since the substrate metal is easily corroded in the electroless plating solution of the displacement type to produce more nickel oxides, the nickel oxide remover is used to remove the nickel oxides on the surface of the substrate metal. Experimental results show that the uniformity of the plating film formed by using the displacement type chemical gold plating solution provided by the invention is good, the grain boundary has no corrosion condition, and the solder wettability is good.
Owner:SHENZHEN FARCIEN APPLIED MATERIALS
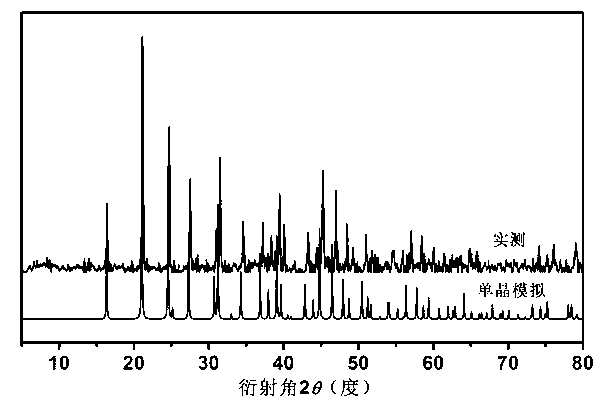
Orthorhombic phase Gd(OH)CO3, preparation method and application thereof
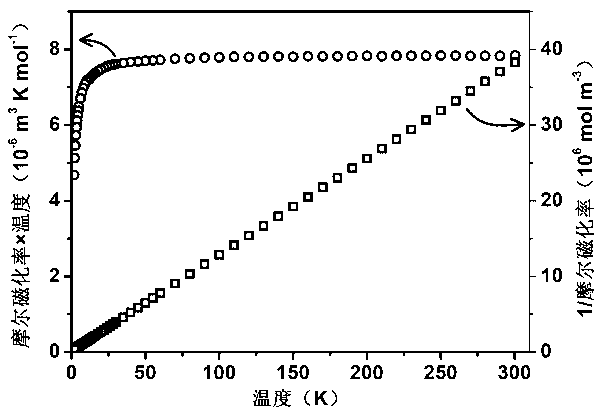
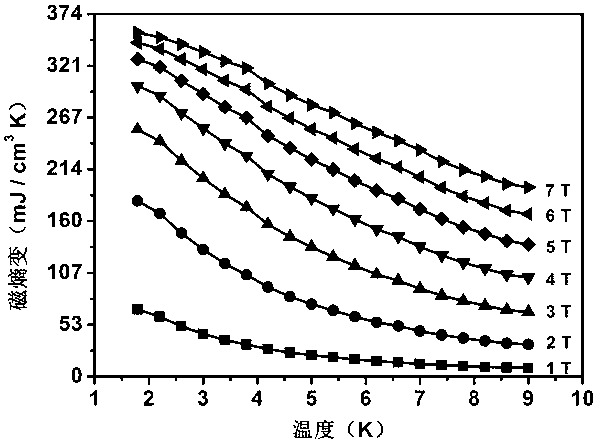
InactiveCN103320859AIncrease flexibilityHigh magnetic entropy changePolycrystalline material growthRare earth metal compoundsCrystal systemMetallurgy
The invention relates to the technical field of the application of gadolinium, and discloses an orthorhombic phase Gd(OH)CO3, and a preparation method and an application thereof. The orthorhombic phase Gd(OH)CO3 is a crystal which belongs to an orthorhombic system, the space group is Pnma, cell parameters are that a=7.0770(7) angstroms, b=4.8730(9) angstroms, c=8.4343(6) angstroms, and alpha=beta=gamma=90 degrees. The preparation method of the orthorhombic phase Gd(OH)CO3 comprises the following steps of mixing a gadolinium salt and malononitrile, adding a solvent to dissolve the mixture, and placing at a constant temperature and obtaining the orthorhombic phase Gd(OH)CO3. The orthorhombic phase Gd(OH)CO3 can replace magnetic refrigeration working mediums for use, and has a prominent magnetocaloric effect.
Owner:SUN YAT SEN UNIV
Treatment method for high-concentration ammonia nitrogen in urine waste water
InactiveCN102633318AAvoid secondary pollutionStrong targetingWater contaminantsWater/sewage treatment by sorptionHigh concentrationPotassium hydroxide
The invention discloses a treatment method for high-concentration ammonia nitrogen in urine waste water. The treatment method comprises the following steps of: smashing and sieving limonite, immersing particles in concentrated hydrochloric acid and a saturate potassium hydroxide methanol solution sequentially, separating the particles under the action of an applied magnetic field to obtain a sediment, adding oxygen malononitrile in the sediment to perform polarization on the sediment, then immersing the sediment in an organic composite denitrifier containing methyl ten-butyl ether, ethylene oxide and the like for 30-50 minutes, washing the sediment for three times with deionized water, and drying the sediment in the presence of nitrogen protection to obtain a magnetic adsorption nanometer material with the organic composite denitrifier. The treatment method for the high-concentration ammonia nitrogen in the urine waste water can be used for realizing the effect of high removing capacity of the combination of the organic composite denitrifier and an air stripping method, and further solving the problem of secondary pollution caused by ammonia emissions in the treating process of the organic composite denitrifier.
Owner:CHANGZHOU YAHUAN ENVIRONMENTAL PROTECTION TECH
Agent for detecting glutathione and its synthesis method and use
InactiveCN106950210AEasy to synthesizeLow costFluorescence/phosphorescenceSynthesis methodsBuffer solution
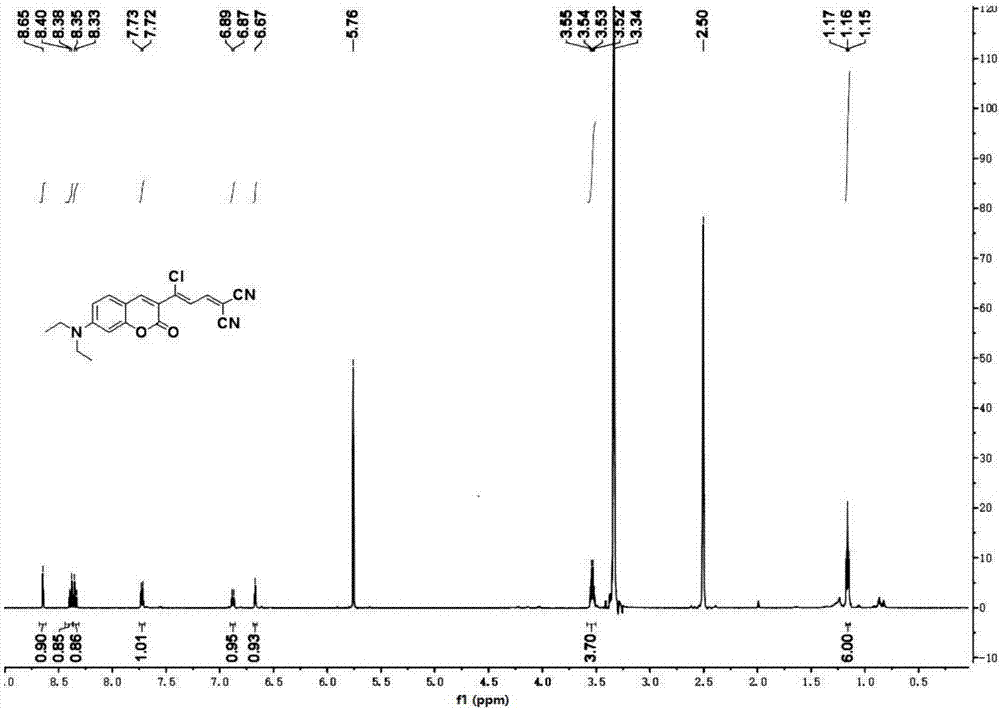
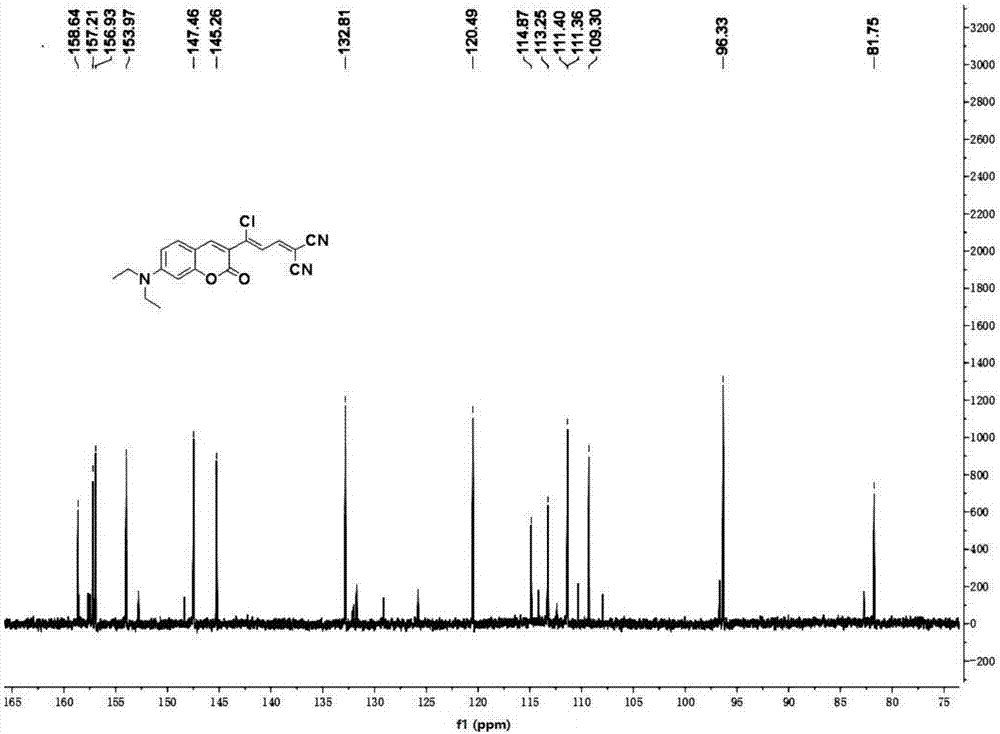
The invention provides an agent and method for detecting glutathione (GSH). The agent is a coumarin derivative (Z)-2-(3-chloro-3-(7-(diethylamino)-2-oxo-2H-chromen-3-yl)allylidene)malononitrile. The method for detection comprises quantitatively detecting the content of glutathione in a PBS buffer solution at pH 7.4 by a fluorescence spectrophotometer. The method has high sensitivity and selectivity to GSH, has simple detection process, sensitivity and a fast detection rate and can produce an accurate detection result.
Owner:SHANXI UNIV
Method for catalytically preparing 4,5-dihydropyran[c]chromene derivative
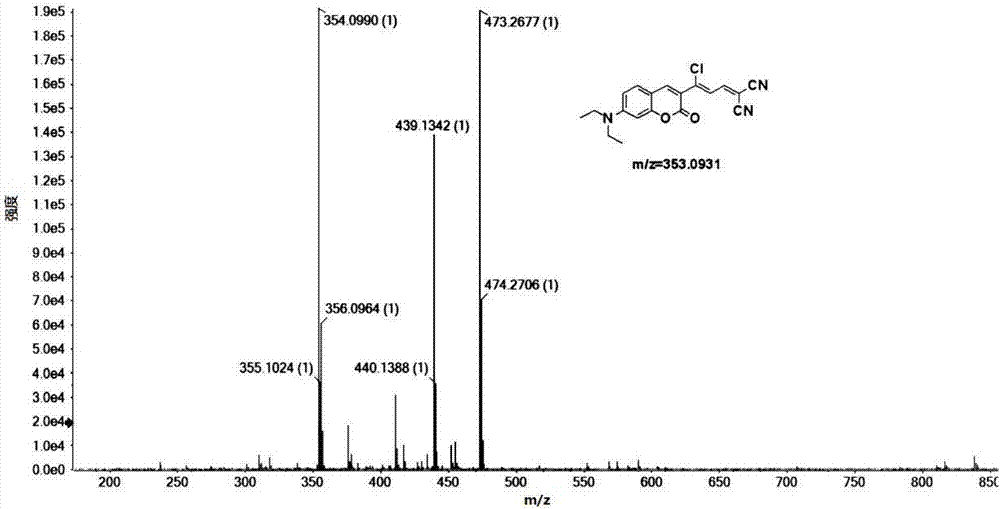
The invention discloses a method for catalytically preparing a 4,5-dihydropyran[c]chromene derivative, and belongs to the technical field of chemical material preparation.In the preparation reaction, the molar ratio of aromatic aldehyde to 4-hydroxycoumarin to malononitrile is 1:1:(1-1.2), the molar weight of an alkaline ionic liquid catalyst is 8%-15% of that of aromatic aldehyde, the volume dose of a 50% ethanol water solution serving as reaction solvent on a milliliter basis is 8-10 times of the molar weight of aromatic aldehyde on a millimole basis, the reflux reaction time ranges from 10 min to 28 min, after the reaction is finished, cooling is conducted to room temperature, extraction filtration is conducted, filter residues are washed with ethyl alcohol and vacuum-dried, and the 4,5-dihydropyran[c]chromene derivative is obtained.Compared with a preparation method adopting other alkaline ionic liquid catalysts, the method for catalytically preparing the 4,5-dihydropyran[c]chromene derivative has the advantages of being high in catalytic activity of the catalyst, free of treatment during the reuse process, high in raw material utilization rate, easy and convenient to operate in the whole preparation process and the like, and large-scale industrial application is convenient.
Owner:日照新睿招商发展有限公司
Preparation method of antipsychotic drug olanzapine
Owner:宁波人健化学制药有限公司
Synthesis process for compound crizotinib
The invention provides a new synthesis method for crizotinib. An atomic economic reaction is adopted to reduce environmental pollution. A high-optical purity raw material is obtained by chiral prolinol induced chiral reduction; a chiral centre is constructed through an SN2 substitution reaction; post-processing and purification difficulties caused by Mitsunobu reaction are overcome. Malononitrile derivative is constructed by adopting a coupling reaction of malononitrile and bromo-pyridinium derivative; N,N-dicarboamide derivatives are obtained by performing aminolysis on N,N-dimethylamine hydrochloride; in the N,N-dicarboamide derivatives, N,N-dimethylamine serving as an easy-to-leave group and hydrazine perform a ring closing reaction to construct a pyrazolone ring, so that an expected final product, namely crizotinib, is obtained. According to the method, though continuous steps are used, the reaction of each step is high, the optical purity is high, and the total yield is also high. In addition, raw materials used in the synthesis method are low in cost and easily obtained; the using amount of a catalyst is small; total cost is easy to control. An operating process is simple and convenient and easy to control, and is suitable for industrial production.
Owner:甘肃皓骏药业有限公司
Benzopyran nitrile-based sulfite fluorescence probe and preparation method thereof
InactiveCN107417654AEasily damagedAvoid damageOrganic chemistryMaterial analysis by observing effect on chemical indicatorBenzopyranLevulinic acid
The invention belongs to the technical field of anion detection and relates to a benzopyran nitrile-based sulfite fluorescence probe and a preparation method thereof. The preparation method comprises the following steps: reacting 2-methylbenzopyran malononitrile and p-hydroxy benzaldehyde to produce 2-(1-hydroxyvinyl phenyl) benzopyran malononitrile, and reacting with acetylpropionic acid to prepare the product. After sulfite ions are added, fluorescence appears at 710nm, the fluorescence peak is enhanced along with increase of the concentration of the sulfite ions, the fluorescence intensity presents a linear relation with the sulfite ion concentration in a range (0-8)*10<-6>M of the concentration of the sulfite ions. The solution color is changed from yellow to blue so as to achieve a colorimetric detection effect. The probe molecule has the characteristic of high interference resistance in a coexistence system of the sulfite ions and other multiple negative ions, and the fluorescence probe is high in selectivity, high in sensitivity and fast in response and can be well applied to sulfite detection in the environment.
Owner:HENAN AGRICULTURAL UNIVERSITY
Popular searches
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com


![Method for synthesizing intermediate 4-amino-3-(4-phenoxy-phenyl)-1H-pyrazolo[3,4-d]pyrimidine of Ibrutinib Method for synthesizing intermediate 4-amino-3-(4-phenoxy-phenyl)-1H-pyrazolo[3,4-d]pyrimidine of Ibrutinib](https://images-eureka.patsnap.com/patent_img/2815104a-272d-435c-ad87-5493807452c7/BSA0000103894400000011.PNG)
![Method for synthesizing intermediate 4-amino-3-(4-phenoxy-phenyl)-1H-pyrazolo[3,4-d]pyrimidine of Ibrutinib Method for synthesizing intermediate 4-amino-3-(4-phenoxy-phenyl)-1H-pyrazolo[3,4-d]pyrimidine of Ibrutinib](https://images-eureka.patsnap.com/patent_img/2815104a-272d-435c-ad87-5493807452c7/BSA0000103894400000012.PNG)
![Method for synthesizing intermediate 4-amino-3-(4-phenoxy-phenyl)-1H-pyrazolo[3,4-d]pyrimidine of Ibrutinib Method for synthesizing intermediate 4-amino-3-(4-phenoxy-phenyl)-1H-pyrazolo[3,4-d]pyrimidine of Ibrutinib](https://images-eureka.patsnap.com/patent_img/2815104a-272d-435c-ad87-5493807452c7/BSA0000103894400000021.PNG)

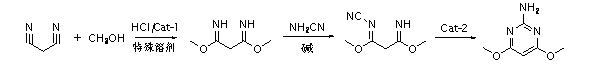
![Green catalytic preparation method of pyrano[4,3-b]pyran derivative Green catalytic preparation method of pyrano[4,3-b]pyran derivative](https://images-eureka.patsnap.com/patent_img/c8c25db8-b820-44f7-b54b-395374a18b6a/BDA0000745322030000021.PNG)
![Green catalytic preparation method of pyrano[4,3-b]pyran derivative Green catalytic preparation method of pyrano[4,3-b]pyran derivative](https://images-eureka.patsnap.com/patent_img/c8c25db8-b820-44f7-b54b-395374a18b6a/BDA0000745322030000031.PNG)
![Green catalytic preparation method of pyrano[4,3-b]pyran derivative Green catalytic preparation method of pyrano[4,3-b]pyran derivative](https://images-eureka.patsnap.com/patent_img/c8c25db8-b820-44f7-b54b-395374a18b6a/FDA0000745322020000011.PNG)
![Method for catalytically preparing 4,5-dihydropyran[c]chromene derivative Method for catalytically preparing 4,5-dihydropyran[c]chromene derivative](https://images-eureka.patsnap.com/patent_img/90327cad-9854-40a4-b7a3-e5c7c6a80dfa/BDA0000991840970000031.PNG)
![Method for catalytically preparing 4,5-dihydropyran[c]chromene derivative Method for catalytically preparing 4,5-dihydropyran[c]chromene derivative](https://images-eureka.patsnap.com/patent_img/90327cad-9854-40a4-b7a3-e5c7c6a80dfa/BDA0000991840970000032.PNG)
![Method for catalytically preparing 4,5-dihydropyran[c]chromene derivative Method for catalytically preparing 4,5-dihydropyran[c]chromene derivative](https://images-eureka.patsnap.com/patent_img/90327cad-9854-40a4-b7a3-e5c7c6a80dfa/FDA0000991840960000011.PNG)