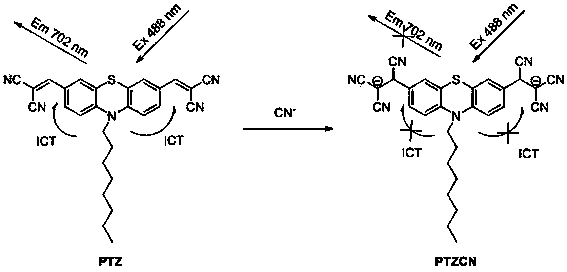
Fluorescent probe as well as preparation method and application thereof
A fluorescent probe and reaction technology, applied in fluorescence/phosphorescence, chemical instruments and methods, luminescent materials, etc., can solve the problems of low probe detection sensitivity, high cost, complex synthesis route, etc., and achieve simple synthesis and fewer synthesis steps. , the effect of high sensitivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] Embodiment 1 (synthesis of probe):
[0044] (1) compound IV-1 Synthesis
[0045]
[0046] In a 50 mL Shrek tube, add the compound III-1 (469 mg, 1.0 mmol), under the protection of argon, inject dry tetrahydrofuran (20 mL), stir at -78 ℃, inject n-butyl lithium in n-hexane solution (2.5 mol?L -1 , 0.96 mL, 2.4 mmol). After stirring for 1 hour, a solution of N-formylmorpholine (344 mg, 3.0 mmol) and tetrahydrofuran (5 mL) was injected. After reacting for 1 hour, it was slowly raised to room temperature, and stirred for 12 hours. Add dilute hydrochloric acid (10 mL, 1 mol?L -1 ) acidified and stirred for 45 minutes. The layers were separated, the organic phase was separated, the aqueous phase was extracted with dichloromethane (20 mL×3), the organic phases were combined, and washed with saturated brine (20 mL). The organic phase was dried with anhydrous sodium sulfate, filtered with suction, the filter cake was washed with dichloromethane (20 mL×2), the filtrat...
Embodiment 2
[0052] Embodiment 2 (probe PTZ Fluorescent detection of cyanide ion):
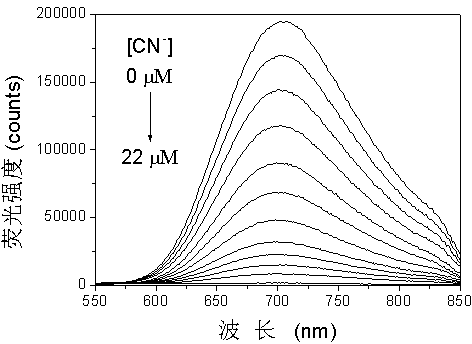
[0053] Dissolve the molecular probe obtained above in a mixed solution of dimethyl sulfoxide-water (volume ratio 9:1), and configure it to 10 μmol?L -1 probe solution. Add 2.5 mL of the probe solution into a 1 cm×1 cm×4 cm stoppered cuvette, and then use a microsyringe to add different concentrations of CN - (sodium salt) after mixing evenly for 2 minutes, test its fluorescence emission spectrum, λ ex = 488 nm, the result is as figure 2 shown. With CN - The red fluorescence of the solution was gradually quenched by the continuous addition of . PTZ to CN - The detection limit is 67 nmol?L -1 , indicating that the probe pair CN - Has high detection sensitivity.
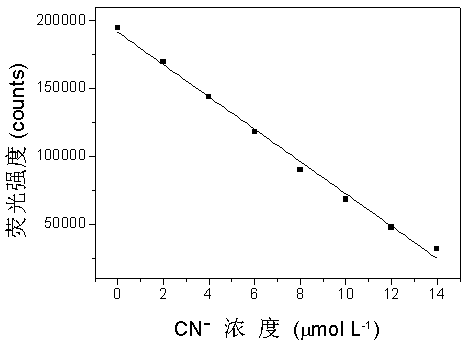
[0054] image 3 expressed in PTZ The concentration is 10 μmol?L -1 Under the condition of the dimethyl sulfoxide-water mixed solution (volume ratio of 9:1), the fluorescence intensity value F of the working solution at 702 nm and the...
Embodiment 3
[0055] Embodiment 3 (probe PTZ to CN - optional):
[0056] Dissolve the molecular probe obtained above in a mixed solution of dimethyl sulfoxide-water (volume ratio 9:1), and configure it to 10 μmol?L -1 probe solution. Add 2.5 mL of probe solution to a 1 cm×1 cm×4 cm stoppered cuvette each time, and then add 22 μmol?L with a microsyringe -1 CN - (sodium salt) and 200 μmol?L -1 Common anions (sodium or potassium salts), such as: F - , Cl - , Br - , I - , NO 2 - , NO 3 - , OAc - , PO 4 3- , HPO 4 2- , H 2 PO 4 - , HCO 3 - , CO 3 2- , SCN - , S 2- , SO 4 - . After uniform mixing for 2 minutes, test its fluorescence emission spectrum, λ ex = 488nm. The fluorescence intensity quenching ratio (I 0 -I) / I 0 (I and I 0 Indicates whether there is an anion or not PTZ The value of the fluorescence intensity at 702 nm) is plotted on the ordinate, and the results are as follows Figure 4 shown. Figure 4 show PTZ to CN - With good selectivity, th...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com