Organic conjugated molecule capable of being processed by solution and application thereof in solar cells
A technology of solar cells and conjugated molecules is applied in the field of solar cells to achieve the effects of high photoelectric conversion efficiency, low HOMO energy level and simple preparation process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
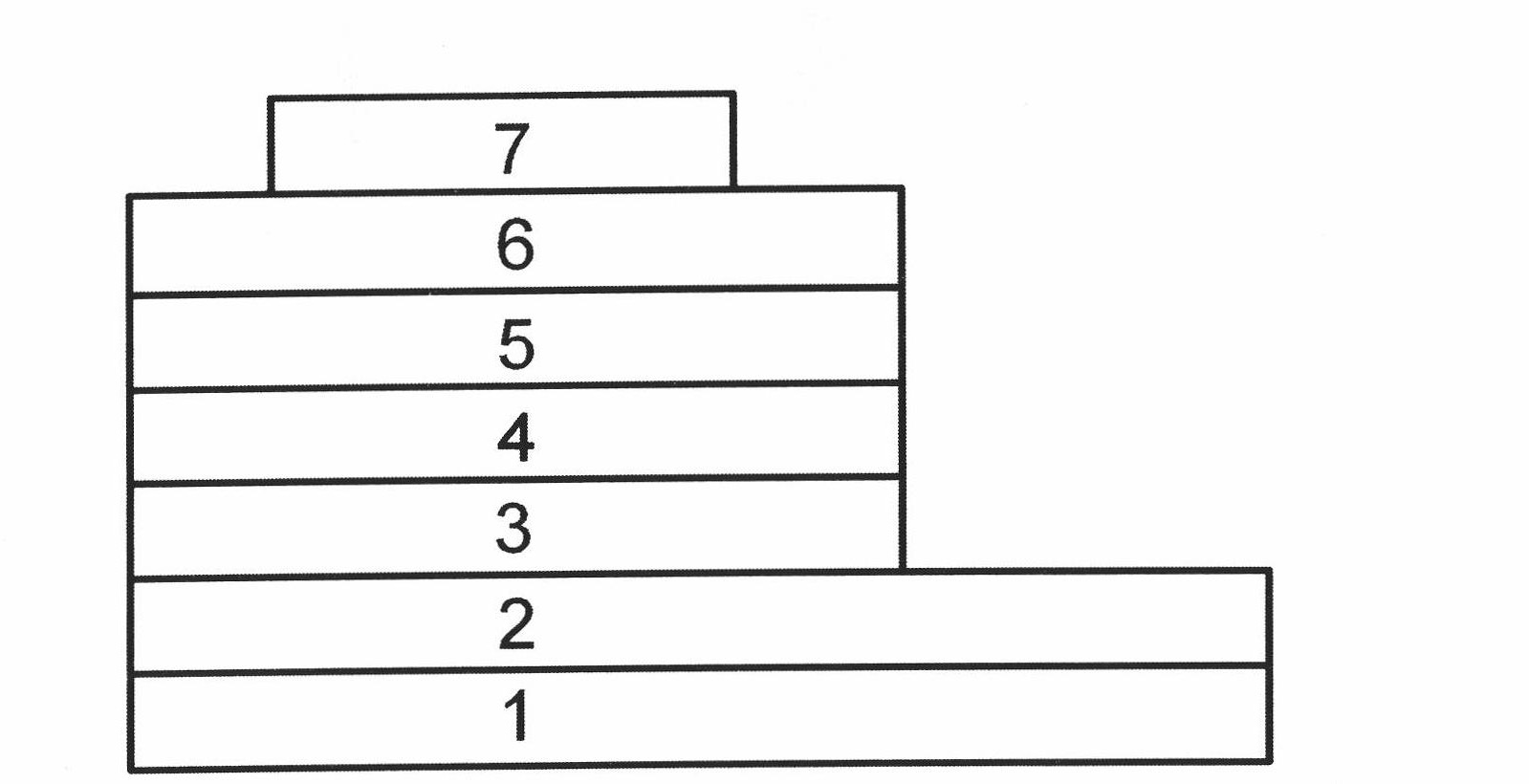
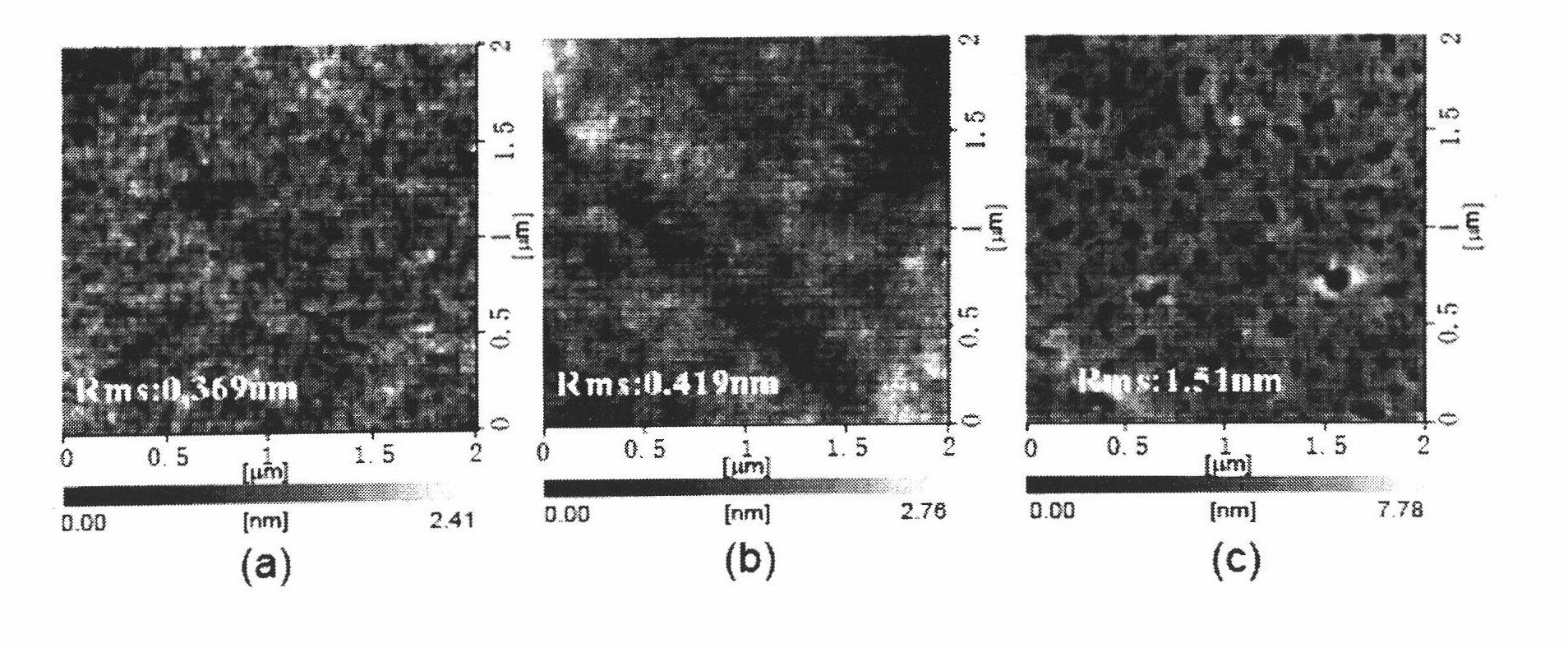
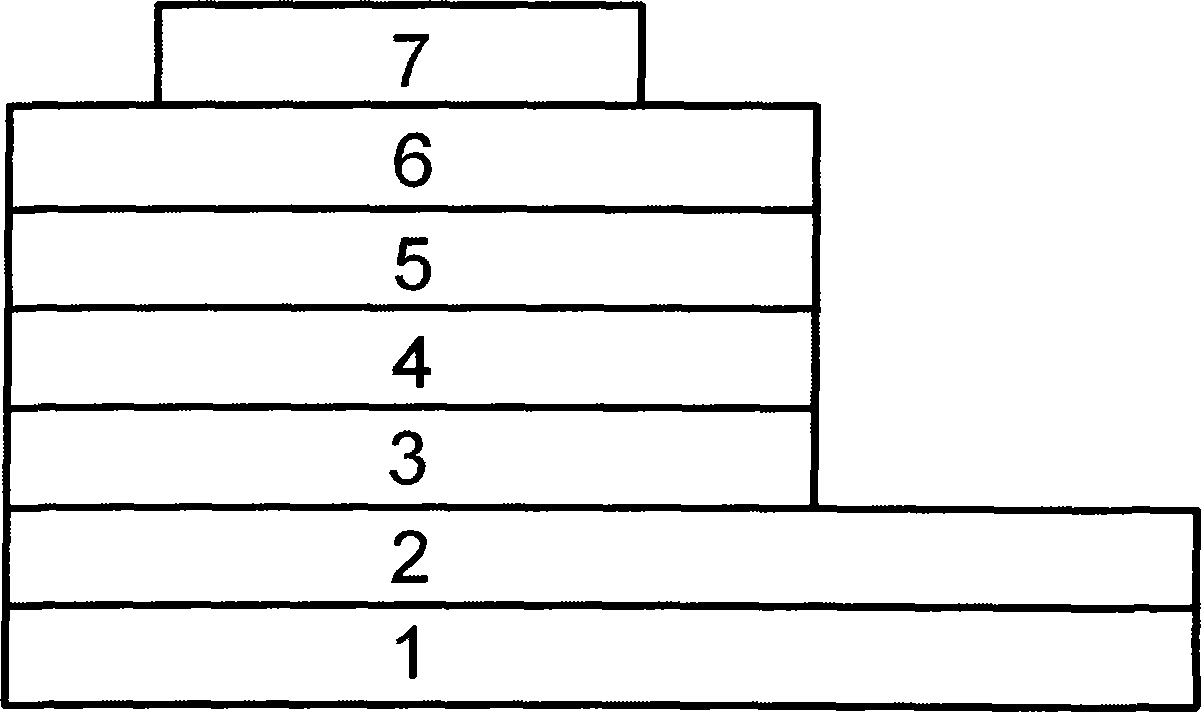
Image
Examples
Embodiment 1
[0034] Embodiment 1: the synthesis of 4TPM
[0035]Put magnesium chips (5.76 g, 0.24 mol) and 20 ml of dry diethyl ether into a three-neck flask to mix, and cool to 0°C. n-Bromohexane (44 ml, 0.312 mol) was added dropwise to the mixed solution, and after the dropwise addition was completed, it was refluxed at 50° C. for 2 hours until the reaction of Mg was complete. Then the reaction temperature was cooled to room temperature, and 1,3-bis(diphenylphosphinopropane)nickel dichloride Ni(dppp)Cl 2 (240 mg, 0.367 mmol) was added to the reaction solution, and 3,4-dibromothiophene (11.36 ml, 0.1 mol) was added dropwise to the solution. After the dropwise addition, react at 50°C for 24h. The reactant was poured into water and extracted with ether, the organic solvents were combined, washed with saturated brine, and then dried over anhydrous magnesium sulfate to remove the solvent. Using petroleum ether as a developing solvent and silica gel as a stationary phase, 18.9 g of light ye...
Embodiment 2
[0042] Embodiment 2: the synthesis of 6TPM
[0043] The synthesis of 6TPM is the same as in Example 1. Only 2,2'-dithiophene-5-tributyltin is used instead of 4,4,5,5-tetramethyl-2-thiophene-1,3,2-dioxaboron. Yield: 80.3%.
[0044] 1 H NMR (500MHz, CDCl3, TMS): δ (ppm) 7.608 (d, 2H, J = 15.6Hz, -vinylic), 7.269 (s, 2H, -Th), 7.224 (d, 2H, -Th), 7.167 (d, 2H, -Th), 7.136 (d, 2H, -Th), 7.054 (m, 2H, -Th), 6.618 (s, 2H, -PM), 6.473 (d, 2H, J=15.3Hz, -vinylic), 2.730(m, 8H, -CH2), 1.573(m, 8H, -CH2), 1.445(m, 8H, -CH2), 1.332(m, 16H, -CH2), 0.921(m, 6H, -CH3), 0.859(m, 6H, -CH3). 13 C NMR(75MHz,CDCl3,TMS):δ(ppm)140.273,138.264,136.727,134.399,134.067,133.136,128.196,127.946,124.904,124.590,124.101,123.966,116.411,115.416,106.503,58.901,31.675,31.610, 31.460, 30.334, 29.500, 29.499, 27.965, 27.721, 22.619, 22.585, 14.015, 13.969. Mass spectrum calculation formula C 60 h 68 N 2 OS 6 Obtain 1025.58; experimental value: 1025.70. Its structural formula is as follows:
[0045]...
Embodiment 3
[0046] Embodiment 3: the synthesis of 8TPM
[0047] The synthesis of 8TPM is the same as in Example 1. Just replace 4,4,5,5-tetramethyl-2-thiophene-1,3,2-dioxabor with 2,2:5,2-trithiophene-5-trimethyltin-trimethyltin . Yield: 72.6%.
[0048] 1 H NMR (500MHz, CDCl3, TMS): δ (ppm) 7.601 (d, 2H, J = 15.6Hz, -vinylic), 7.252 (m, 2H, -Th), 7.202 (m, 2H, -Th), 7.134 (m, 8H, -Th), 7.042 (m, 2H, -Th), 6.605 (s, 2H, -PM), 6.460 (d, 2H, J=15.3Hz, -vinylic), 2.733 (m, 8H, -CH2), 1.587(m, 8H, -CH2), 1.460(m, 8H, -CH2), 1.323(m, 16H, -CH2), 0.927(m, 6H, -CH3), 0.864(m, 6H, -CH3). 13 C NMR(75MHz,CDCl3,TMS):δ(ppm)158.021,155.199,147.211,147.119,140.325,137.928,136.925,136.888,135.445,134.542,134.048,133.028,128.167,127.923,127.294,124.717,124.571,124.419, 124.035, 123.883, 116.482, 106.558, 58.964, 31.696, 31.638, 31.493, 30.334, 29.688, 29.537, 29.482, 28.033, 27.746, 22646, 14.050, 13.992 C. Mass spectrum calculation 68 h 72 N 2 OS 8 Obtain 1189.83; experimental value: 1189.20. ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com