Fluorescent molecular probe for detecting fluoride ions in aqueous solutions as well as synthesis method and application thereof
A technology of fluorescent molecular probes and fluorine ions, which is applied in the field of chemical analysis and detection, can solve the problem of short emission wavelength of the test system, and achieve the effects of wide detection range, high sensitivity and good selectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Embodiment 1: the preparation of compound 2
[0030]In a 50 mL single-neck round bottom flask, add 1,4-diethyl-1,2,3,4-tetrahydro-7-hydroxyquinoxalin-6-al (23.4 mg, 0.1 mmol), 4-di Dissolve methylaminopyridine (16 mg, 0.13 mmol) and a drop of triethylamine in 10 mL of dichloromethane and stir at room temperature for 5 minutes. Tert-butyldimethylsilyl chloride (20 mg, 0.13 mmol) was added to the above reaction system, followed by stirring at room temperature for 1 hour. The reaction solution was poured into 30 mL of water to quench the reaction. Extracted with 30mL of dichloromethane, separated the organic phase and dried it with anhydrous sodium sulfate, distilled off the solvent, and purified by column chromatography (petroleum ether: ethyl acetate = 3:1) to obtain 30 mg of the product as a yellow liquid (yield: 86%).
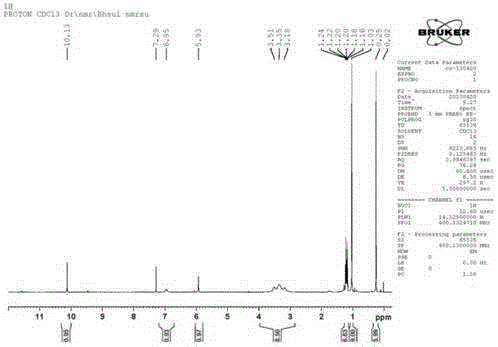
[0031] 1 H NMR (400 MHz, CDCl 3 ) δ 10.14 (d, J = 7.2 Hz, 1H), 6.99 (d, J = 27.2 Hz, 1H), 5.91 (d, J = 15.7 Hz, 1H), 3.35 (t, J = 65.5 H...
Embodiment 2
[0032] Embodiment 2: Preparation of molecular fluorescent probe
[0033] In a 50 mL single-necked flask, compound 2 (34.8 mg, 0.1 mmol) and malononitrile (6.6 mg, 0.1 mmol) were dissolved in 5 mL of ethanol, 1 drop of piperidine was added, and reacted at room temperature for 30 minutes. The reaction solution was poured into water, extracted with dichloromethane, dried over anhydrous sodium sulfate, and separated by column chromatography (petroleum ether / dichloromethane=1:1) to obtain 21 mg of the product (yield: 53%) which was the probe compound.
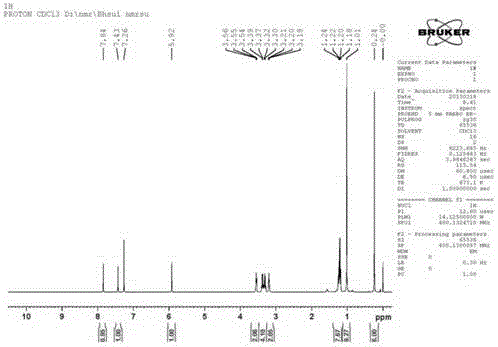
[0034] 1 H NMR (400 MHz, CDCl 3 ) δ 7.84 (s, 1H), 7.43 (s, 1H), 5.92 (s, 1H), 3.59 – 3.52 (m, 2H), 3.35 (dq, J = 25.8, 7.1 Hz, 4H), 3.23 – 3.17 (m, 2H), 1.35 – 1.13 (m, 6H), 1.01 (s, 9H), 0.24 (s, 6H).
Embodiment 3
[0035] Example 3: Application of fluorescent molecular probes for detection of fluoride ions
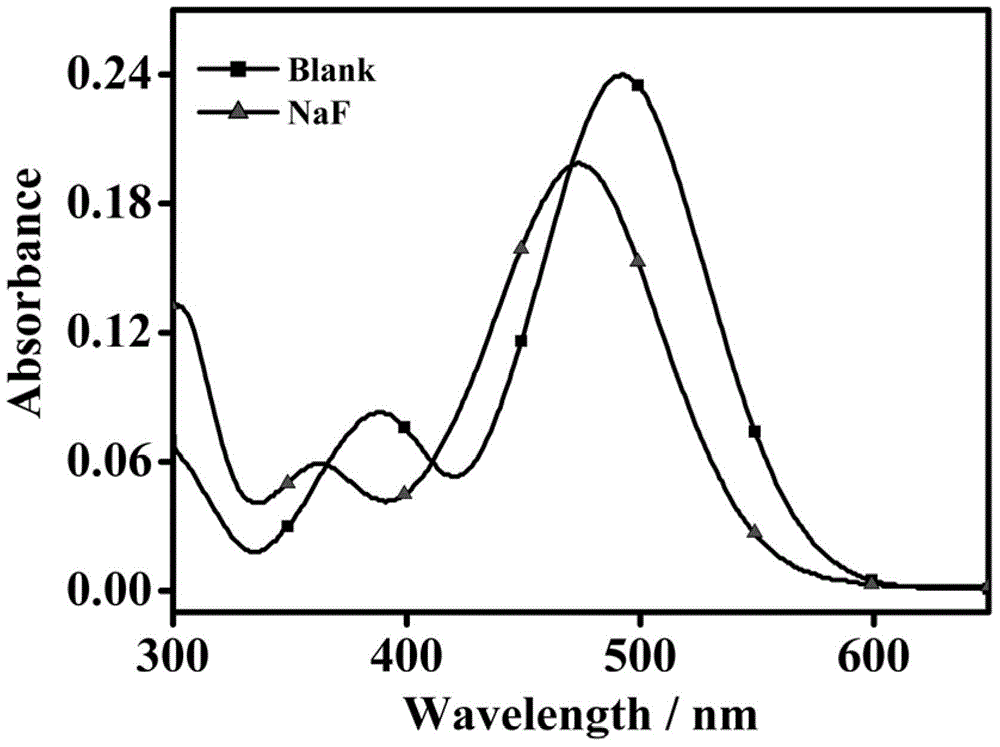
[0036] Dissolve the probe in 30% acetonitrile:water solution at a concentration of 10 μM, add 1 mM sodium fluoride solution, and test the ultraviolet absorption spectrum; dissolve the probe in 30% acetonitrile:water solution at a concentration of 10 μM, first , adding different concentrations of sodium fluoride solution, additionally, adding different test substances (1 mM chloride ion, bromide ion, iodide ion, tetrabutylammonium cyanide, nitrate, hydrogen sulfate , perchlorate, acetate, thiocyanate, azide, cysteine, bovine serum albumin, carbonate, sulfate, reduced glutathione), and finally, in 1 mM coexisting substances (chloride ion , bromide ion, iodide ion, tetrabutylammonium cyanide, nitrate, hydrogen sulfate, perchlorate, acetate, thiocyanate, azide, cysteine, bovine serum albumin, carbonate, sulfuric acid root, reduced glutathione), 1 mM sodium fluoride solution was added...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com