Application of high selectivity reaction type fluorescence probe for detecting hydrogen sulfide
A fluorescent probe, high selectivity technology, applied in fluorescence/phosphorescence, luminescent materials, organic chemistry, etc., can solve the problems of limited detection methods, inability to detect with H2S, complicated pretreatment process, etc., and achieve simple and easy synthesis process Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] Example 1 Synthesis of 2-{(6-(dimethylamino)-3-(2,4-dinitrophenoxy)naphthaleneacetamide-2-)methylene}malononitrile
[0039] Take a 50ml eggplant-shaped bottle, add compound (3) (50 mg, 0.13 mmol), piperidine (0.5 ml), glacial acetic acid (0.5 ml) into it, add 12 ml of toluene, stir to dissolve at room temperature, and then add propane Dinitrile (80 mg, 1.2 mmol), under nitrogen protection, was stirred at room temperature for 1 h, and the reaction was completed. Add 25 ml of ice water to an eggplant-shaped bottle, wash the layers with a separatory funnel, keep the organic phase, then wash with 50 ml of water at 50°C three times, remove excess malononitrile, keep the organic phase, wash with anhydrous magnesium sulfate Dry and filter, distill toluene off under reduced pressure to obtain a dark red sticky solid, beat with 1 ml of petroleum ether, filter, rinse the filter cake with 5 ml of 50°C hot water, and dry to obtain a red solid powder with a yield of 90%. 1 HNMR (40...
Embodiment 2
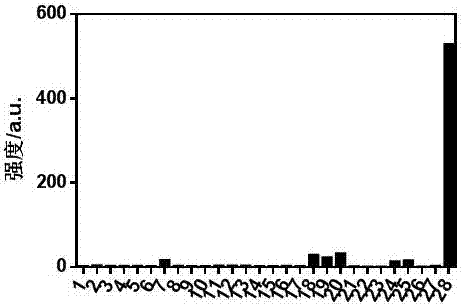
[0040] Embodiment 2 probe is to the selectivity of hydrogen sulfide
[0041] The reaction was carried out in acetonitrile:PBS (9:1, v:v, 25mM) system. In a system with a final volume of 3mL, a certain amount of probe molecules were added, and then different cations, anions, amino acids and Solutions of other small molecules. After 60 min of reaction, the fluorescence intensity of the system was measured. (See figure 1 )
Embodiment 3
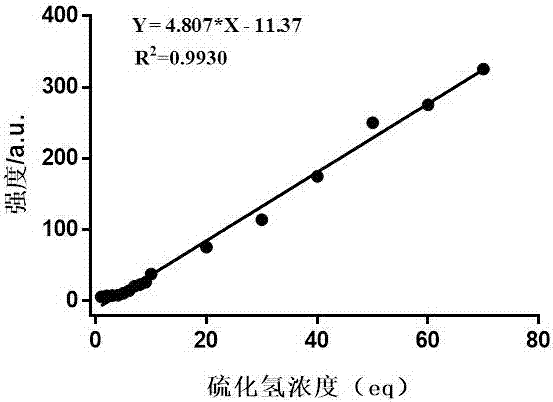
[0042] Embodiment 3 probe is measured to the concentration curve of hydrogen sulfide linear reaction
[0043] The reaction was carried out in the system of acetonitrile:PBS (9:1, v:v, 25mM). Hydrogen sulfide of different concentrations was added to the system with a final volume of 3mL, and the probe (final concentration 20 mM) was added to react for 60min, and the fluorescence intensity was measured. . (See figure 2 ). The linear curve equation is Y = 4.807 * X -11.37 (R 2 = 0.993), where Y represents the fluorescence intensity at 625 nm, and X the hydrogen sulfide concentration.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com