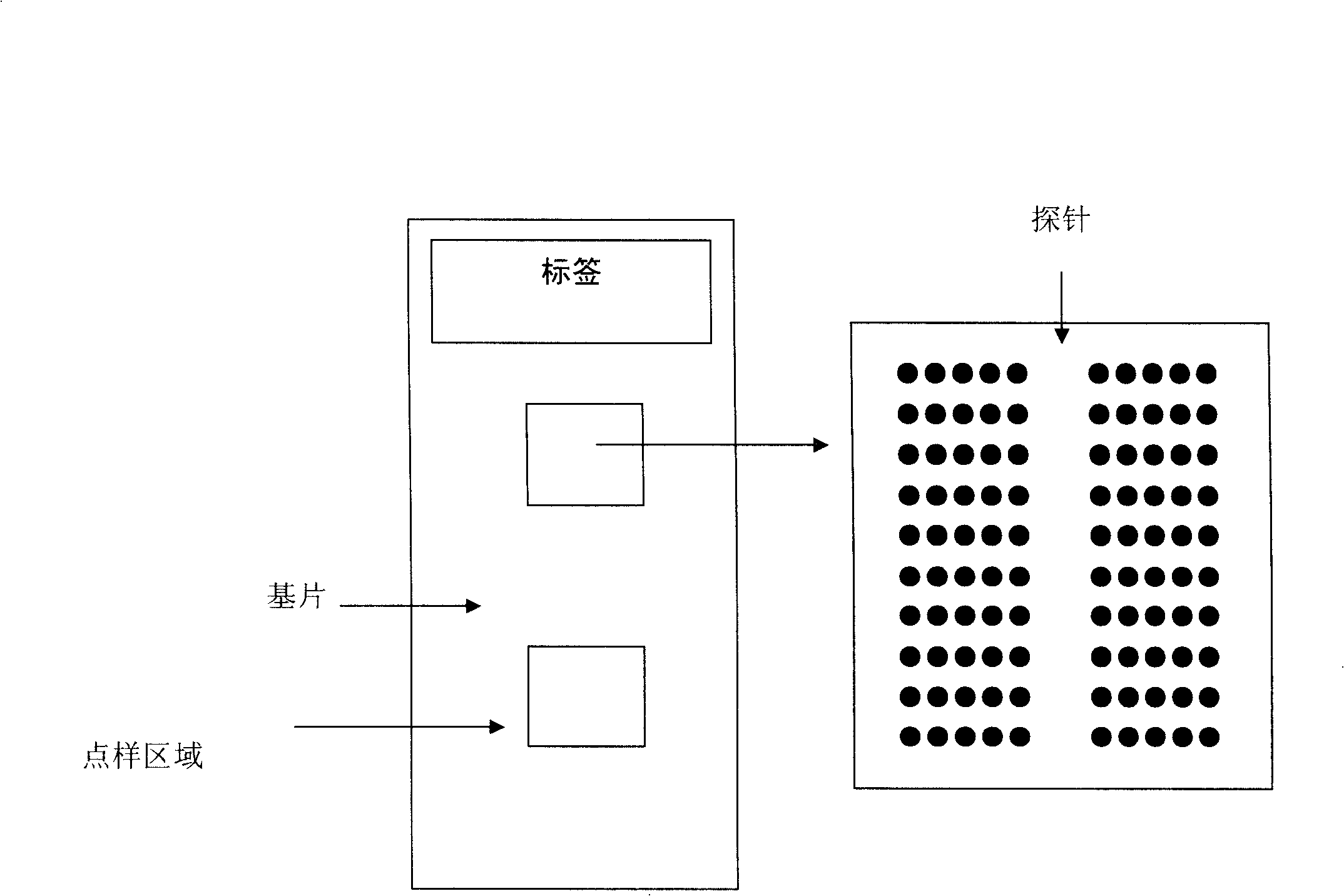
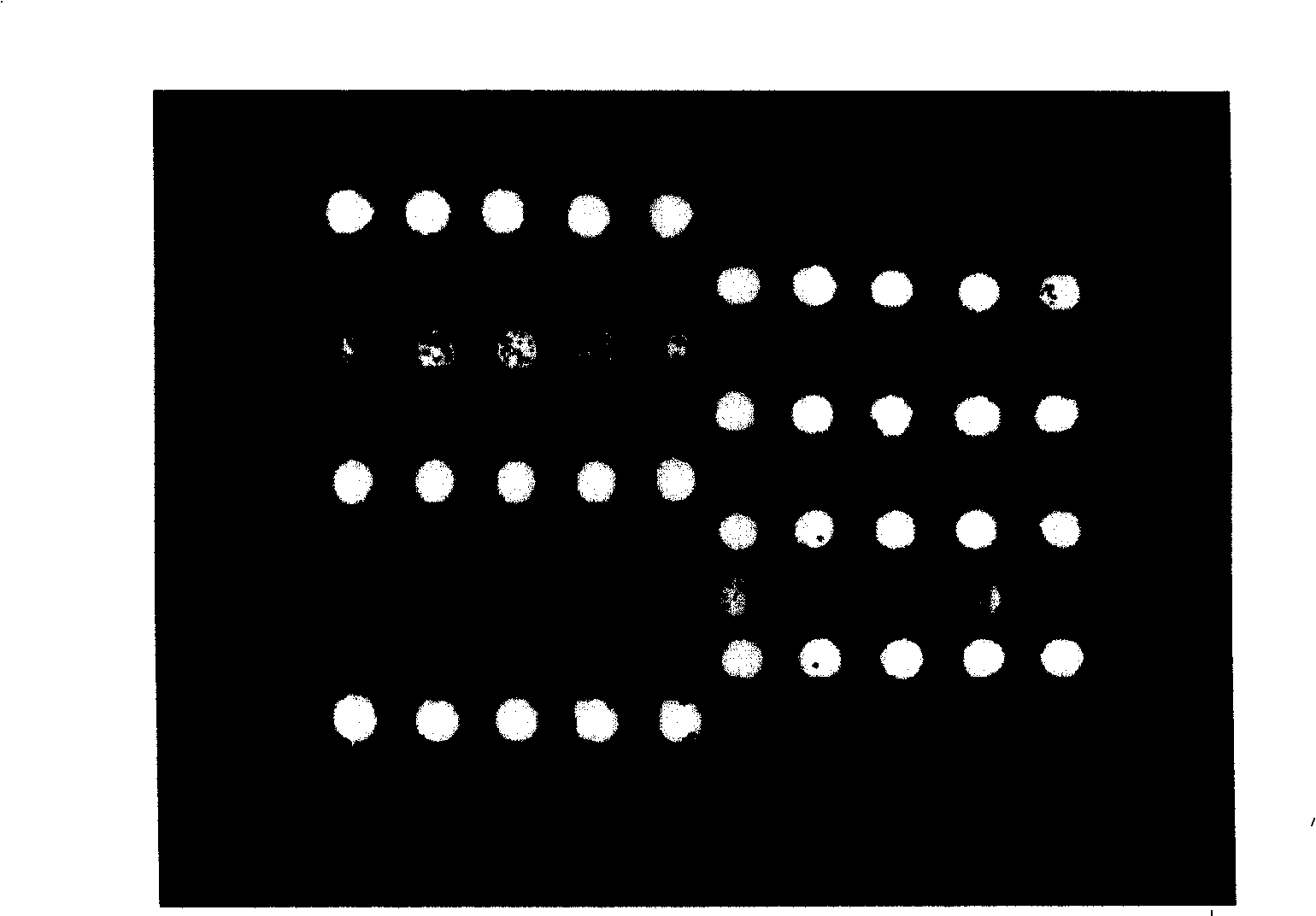Gene chip for detection of hyperpiesis individual medicine correlated gene mutation and uses thereof
A gene chip and high blood pressure technology, applied in the field of gene chips, can solve the problems of low frequency of occurrence of ADRB1, no necessity of detection, and influence on the accuracy of detection results, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0076] Example 1: Preparation of gene chip for detection of gene mutations related to individualized medication for hypertension
[0077] 1. The main raw and auxiliary materials and equipment for preparation (as shown in Table 4)
[0078] Table 4
[0079] Reagents and equipment
Origin
Reagent purity / concentration
Blank ultra-flat film
Shanghai Baiao Technology Co., Ltd.
/
Aminosilane reagent
Acros (United States)
99wt.%
Acros (United States)
25wt.%
95% ethanol
Shanghai Chemical Plant
Analytically pure
Glacial acetic acid
Shanghai Chemical Plant
Analytically pure
NaBH4
Shanghai Chemical Plant
Analytically pure
Na 2 HPO 4
Shanghai Chemical Plant
Analytically pure
KH 2 PO 4
Shanghai Chemical Plant
Analytically pure
Na 2 CO 3
Shanghai Chemical Plant
Analytically pure
NaHCO 3
Shanghai Chemical Plant ...
Embodiment 2
[0102] Example 2: Using the gene chip prepared in Example 1 to treat five hypertension drugs: CYP2C9*3, ADRB1 (1165G>C), AGTR1 (1166A>C), CYP2D6*10, and ACE (I / D) Genetic mutations are detected.
[0103] From August 2005 to June 2006, clinical verification was carried out in the Third Xiangya Hospital of Central South University, the First Affiliated Hospital of Sun Yat-sen University, and Jiangxi Provincial People's Hospital. A total of 1073 samples were tested, and the samples were human peripheral whole blood. For hypertension outpatients and inpatients and other random populations in cities and surrounding areas where each clinical verification unit is located. The clinical verification work is carried out in the clinical verification unit. The clinical verification method is the control method, and the PCR-RFLP genotyping method used routinely in clinical practice and the direct sequencing method as the gold standard are used as the control, and the typing test is performed o...
Embodiment 3
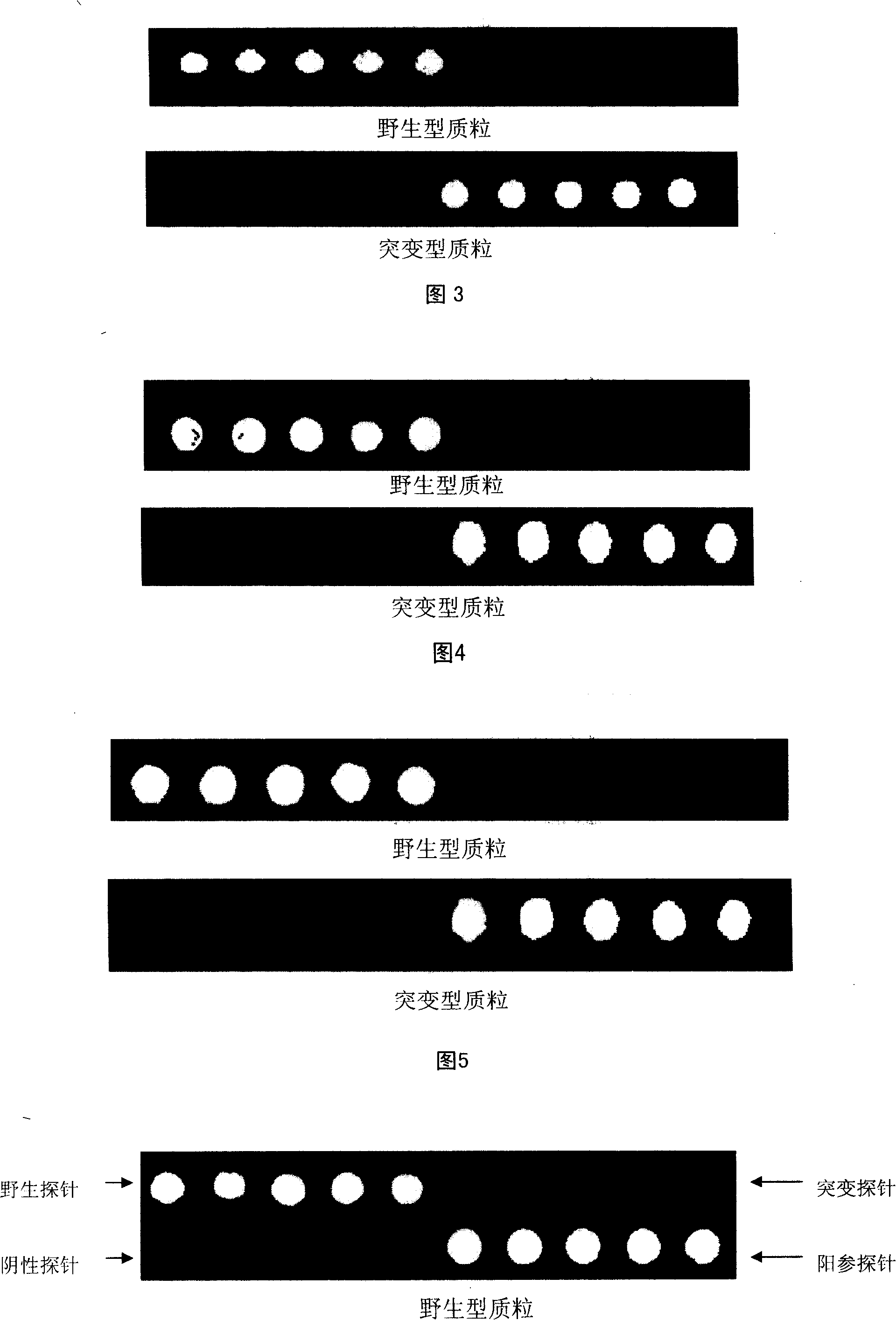
[0150] Example 3: Verify the consistency of the gene chip detection results prepared by the forward probe and the reverse probe
[0151]The present invention can synthesize a set of forward probes (including detection probes and reference probes) and a set of reverse probes (including detection probes and reference probes) for each site. The effect of forward probe and reverse probe in chip detection is the same, provided that the forward probe and the corresponding reverse probe are consistent in length and base arrangement, and each probe at the same detection site The needle direction should be the same, and different detection sites can be randomly selected to synthesize normal phase or reverse probes to form an array. In addition, the PCR primers at each position label the front primer (F primer) when the probe is in the forward direction, and label the rear primer (R primer) when the probe is in the reverse direction. This example aims to verify the consistency of the detect...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com