Agent for detecting glutathione and its synthesis method and use
A technology for glutathione and a synthesis method, which is applied to a reagent for detecting glutathione and its synthesis. The application of the reagent in the detection of glutathione can solve the problems of less reagents and the like, and achieve high sensitivity and selection. The effect of low cost of detection system and simple detection method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
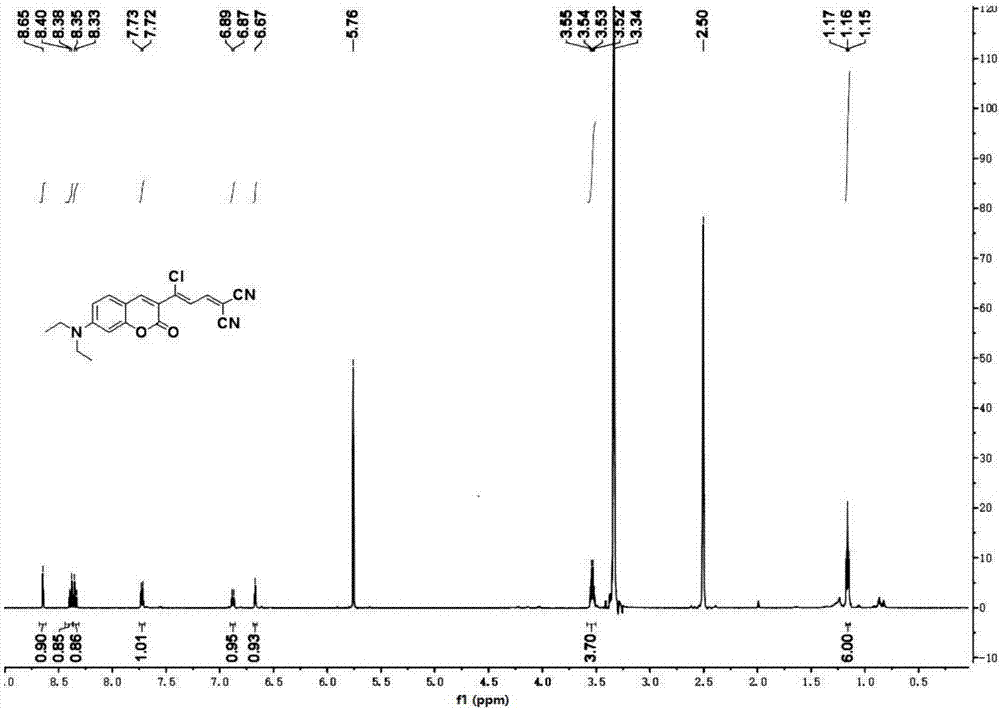
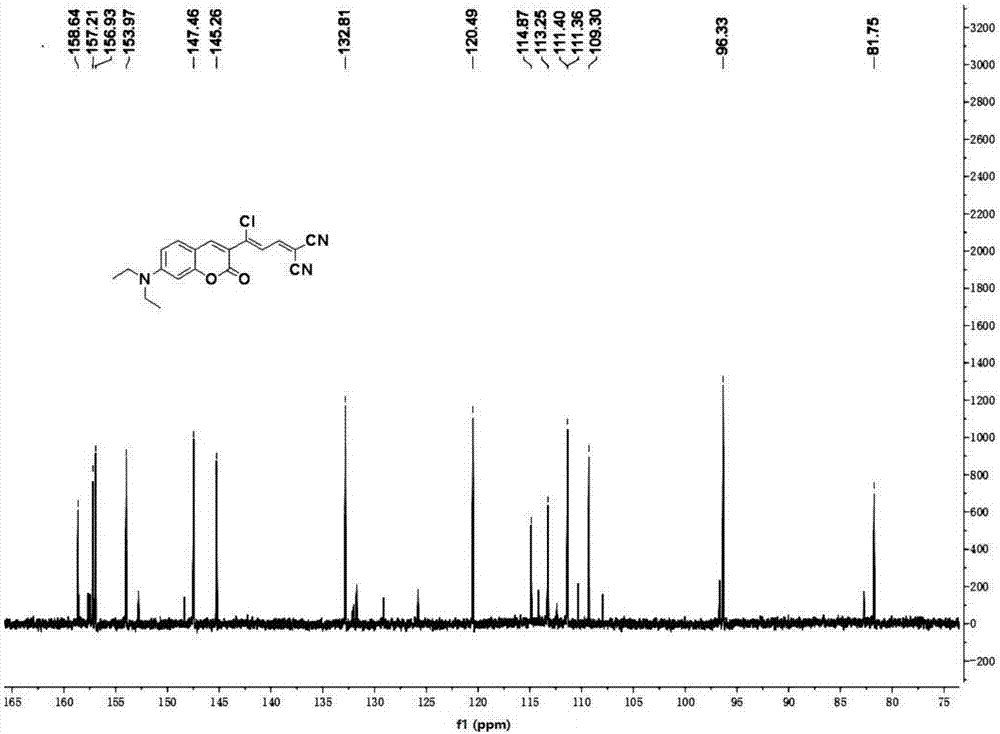
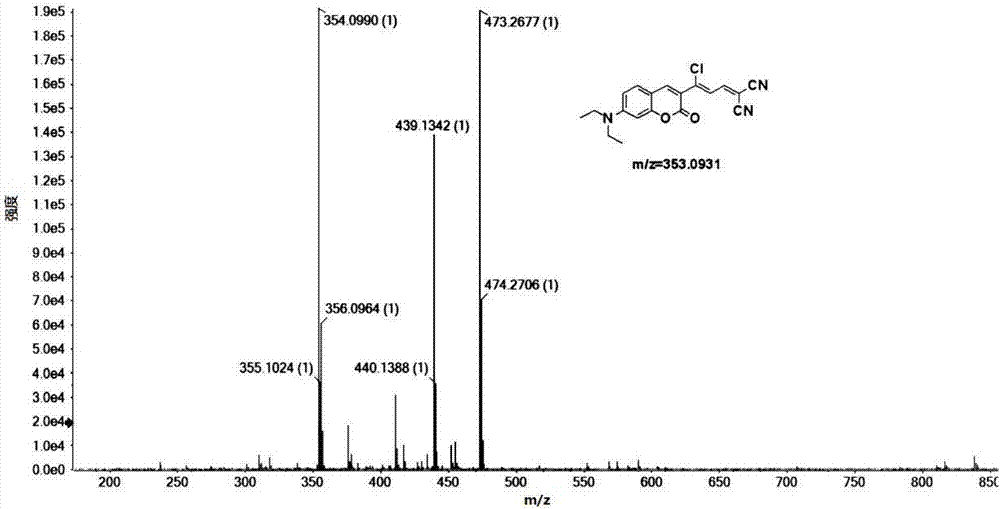
[0031] The preparation of embodiment 1 (Z)-2-(3-chloro-3-(7-(diethylamino)-2-oxygen-2 hydrogen-chromene-3-yl) allyl) malononitrile and characterization
[0032] Slowly add 10 mL of phosphorus oxychloride dropwise to 10 mL of DMF solution under ice bath, stir for half an hour, add 3-(diethylamino)phenol (4.95 g, 20 mmol) dissolved in DMF, and reflux at 75°C for 4 h; After the reaction was completed, the reaction solution was poured into 60mL of ice water and washed with Na 2 CO 3 The pH of the aqueous solution was adjusted to neutral, and a brown solid was precipitated, filtered with suction, washed with water, and finally recrystallized with ethanol to obtain 4-(diethylamino)-2-m-hydroxybenzaldehyde;
[0033] 4-(diethylamino)-2-m-hydroxybenzaldehyde (1.16g, 6mmol) and ethyl acetoacetate (1.17g, 9mmol) were refluxed in ethanol for 10 hours under the catalyst of 0.2mL piperidine, concentrated and cooled Afterwards, the yellow solid obtained by distillation under reduced press...
Embodiment 2
[0038] Prepare a PBS buffer solution with a pH of 7.4 and a concentration of 10 mM, and prepare 2 mM (Z)-2-(3-chloro-3-(7-(diethylamino)-2-oxo-2hydro-chromene- 3-yl) allyl) malononitrile solution; put 2mL of PBS-DMSO (1:1, pH7.4) solution and 5μL (Z)-2-(3-chloro-3-(7-( Add the DMSO solution of diethylamino)-2-oxo-2hydrogen-chromen-3-yl)allyl)malononitrile to a clean fluorescent cuvette, take the GSH solution, and gradually add it in a small amount Add the sample into the cuvette, and detect it on the fluorescence spectrophotometer while adding the sample. With the addition of GSH, the fluorescence intensity at 505nm gradually increases. Fluorescence emission map see Figure 4 .
Embodiment 3
[0040] Prepare a PBS buffer solution with a pH of 7.4 and a concentration of 10 mM, and prepare 2 mM (Z)-2-(3-chloro-3-(7-(diethylamino)-2-oxo-2hydro-chromene with DMSO) -3-yl) allyl) malononitrile solution; in 22 fluorescent cuvettes, add 2 mL of PBS-DMSO (1:1, pH 7.4) solution and 5 μL of reagent in DMSO solution, and then respectively Add 30 μL of GSH, and 300 μL of various analytes: Cys, Hcy, Ala, Arg, Asp, Gln, Glu, Gly, His, lle, Leu, Lys, Met, Phe, Pro, Ser, Thr, Tyr, Trp, Val and Asn are detected on a spectrofluorometer, and a histogram of the relative fluorescence intensity at 505nm corresponding to different analytes is drawn, (see Figure 5 ). GSH changed the fluorescence intensity of the reagent from 16 to 2983, and other analytes basically did not cause changes in the fluorescence intensity of the reagent.
[0041] It has been proved by experiments that other analytes do not interfere with the determination of GSH by the system.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com