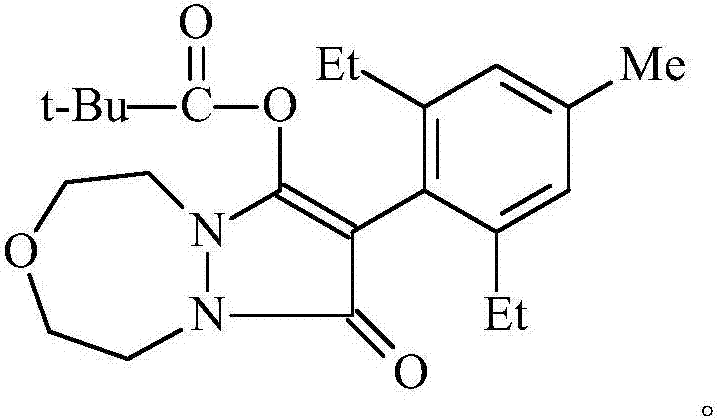
Preparation method of pinoxaden
A technology for pinoxaden and diazepine, which is applied in the field of preparation of technical compounds, can solve problems such as high cost and high environmental pressure, and achieve the effects of reducing the discharge of waste acid, reducing environmental pollution and improving safety
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
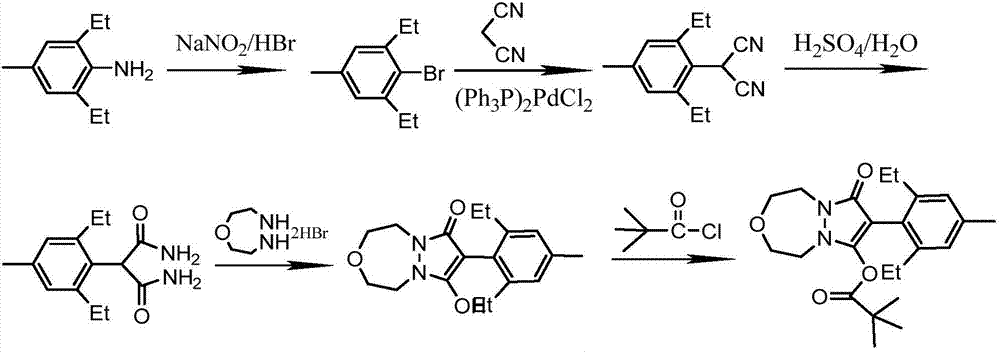
Embodiment 1
[0040] To prepare pinoxaden, the specific steps are as follows:
[0041] 1. Synthesis of 4-methyl-2,6-diethylbromobenzene
[0042]
[0043] Add 65.2g (0.4mol) of 2,6-diethyl-4-methylaniline to 280g of hydrobromic acid solution with a mass fraction of 40%, cool to 0-5°C, and add sodium nitrite aqueous solution drop by drop (33.1g, 0.48mo1 sodium nitrite dissolved in 100mL water), after dripping, stir for 30min and then add 26.2g (0.2mo1) cuprous bromide, heat to 60°C for 4h, TCL tracking the reaction process, the reaction is over Finally, the mixture was poured into 250 mL of ice water, extracted three times with dichloromethane, then dried with anhydrous sodium sulfate and rotary evaporated, concentrated to obtain 92.36 g of crude product, and the fraction at 102-106 °C / 5 mmHg was collected by vacuum distillation to obtain 81.07 g product. Yield 89.2%.
[0044] 1 H NMR (CDCl 3 ) test data are: 1.22(t, 6H); 2.30(s, 3H); 2.85(m, 4H); 7.10(s, 2H).
[0045] 2. Synthesis o...
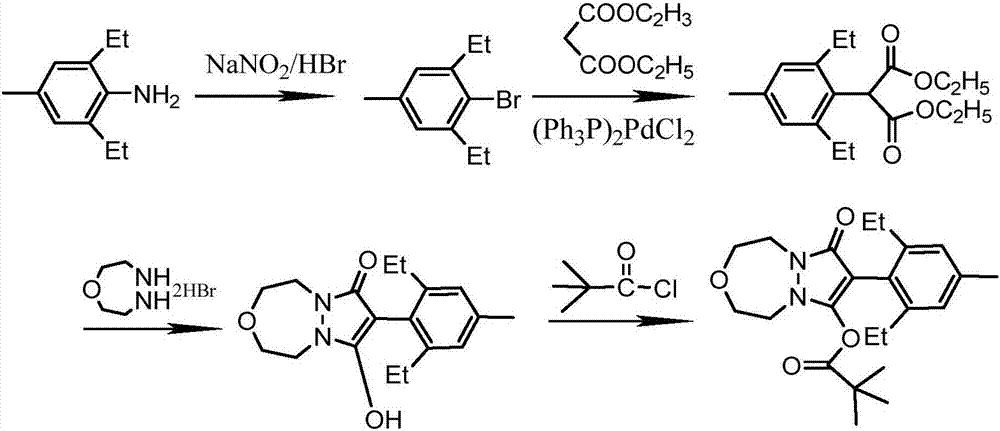
Embodiment 2
[0059] To prepare pinoxaden, the specific steps are as follows:
[0060] 1. The same method as in Example 1 was used to prepare 4-methyl-2,6-diethylbromobenzene.
[0061] 2. Synthesis of 2-(2,6-diethyl-4-methylbenzene)diethyl malonate
[0062]
[0063] Add 32.0g (0.2mol) of diethyl malonate, 16g (0.4mol) of sodium hydroxide and 100mL of N-methylpyrrolidone into a 250mL four-necked reaction flask equipped with a stirrer, a thermometer, and a condenser. Pass nitrogen protection, heat to 100-110°C, and react for 2 hours. Then add 22.7g (0.1mol) of 2,6-diethyl-4-methylbromobenzene and 1.2g of cuprous bromide, and react at 120-130°C for 10h. Cool, then remove the solvent under negative pressure -0.095MPa, 90°C, add 200g of water, adjust pH=3 with hydrochloric acid, extract with 3×80mL ethyl acetate, wash with 3×150mL water, concentrate, add 10mL n-hexane to freeze Crystallized, filtered, and washed with n-hexane to obtain 22.9 g of light yellow solid with a yield of 75%.
[...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com