Benzopyran nitrile-based sulfite fluorescence probe and preparation method thereof
A technology of methylchromene malononitrile and benzopyranonitrile, which is applied in the field of anion detection, can solve the problem of fewer types, and achieve the effects of high selectivity, strong penetration, and strong anti-interference ability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] 1. Experimental part
[0046] Reagents and materials: All reagents were from Shanghai Jingchun Reagent Co., Ltd.
[0047] 1. Preparation of Intermediate I
[0048] In a 100mL round bottom flask, add 208mg (1mmol) 2-methylchromene malononitrile, 144mg (1.2mmol) p-hydroxybenzaldehyde, 20mL acetonitrile, 0.8×10 -2 mmol piperidine, heated to reflux for 6 hours, cooled, and the solvent was removed with a rotary evaporator, the residue was extracted with dichloromethane and 2% sodium bicarbonate solution, and the organic layer was washed with anhydrous Na 2 SO 4 After drying, filtering, and concentration, the residue was passed through a silica gel column (PE:EtOAc=3:1) to obtain 166 mg of an orange-red solid with a yield of 53%.
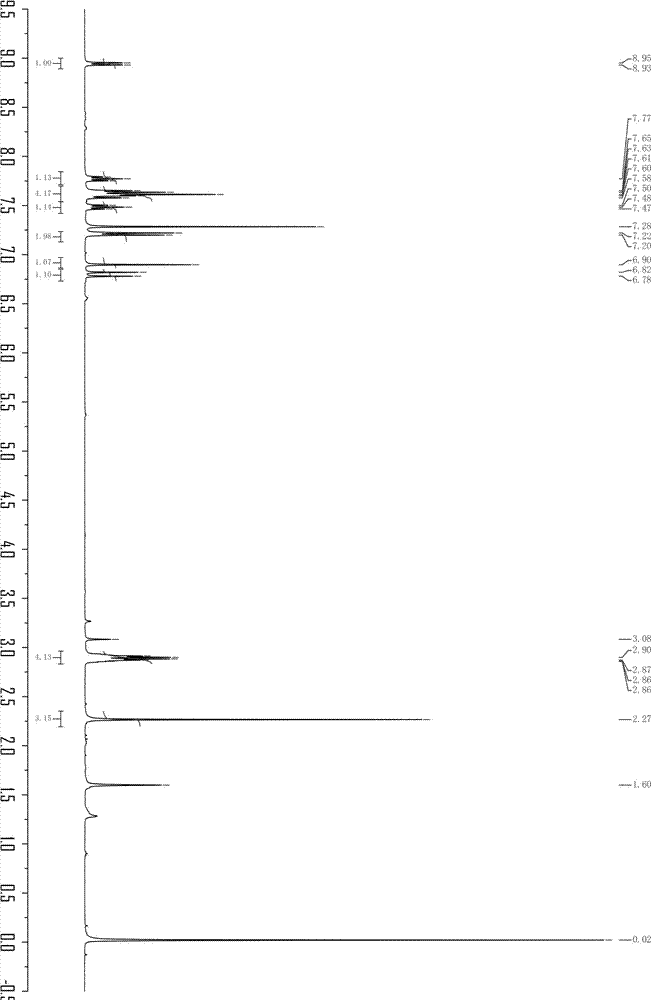
[0049] 1 H-NMRδ (400MHz, DMSO, ppm): 10.15(s, 1H), 8.686-8.707(d, J=8.4Hz, 1H), 7.871-7.910(t, 1H), 7.742-7.763(d, J=8.4 Hz, 1H), 7.557-7.673 (m, 4H), 7.211-7.251 (t, J=16Hz, 1H), 6.835-6.908 (t, J=8.0Hz, 3H).
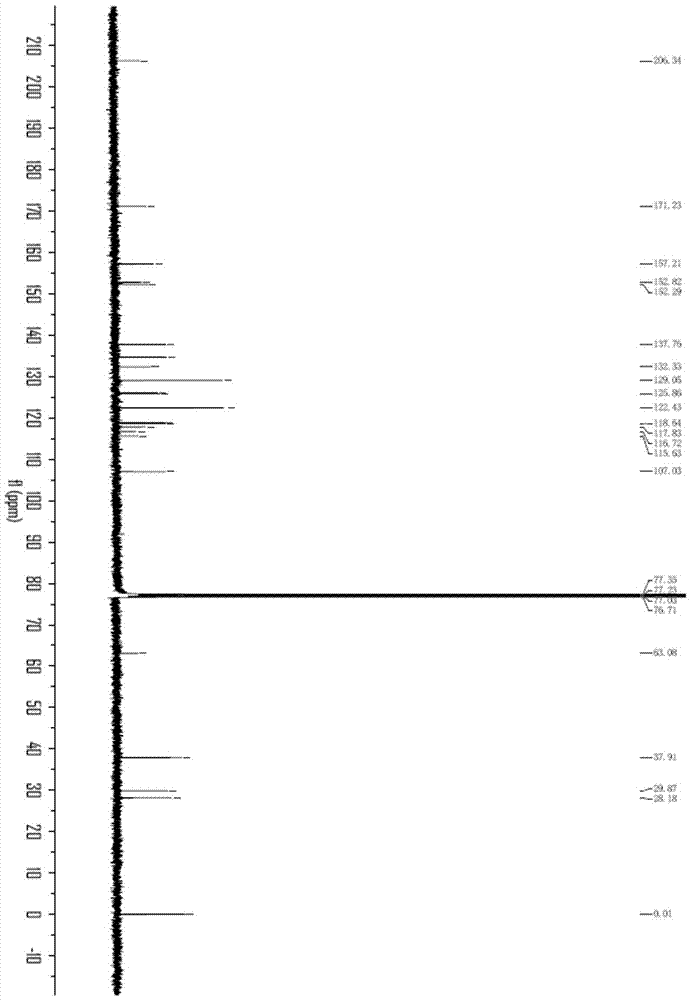
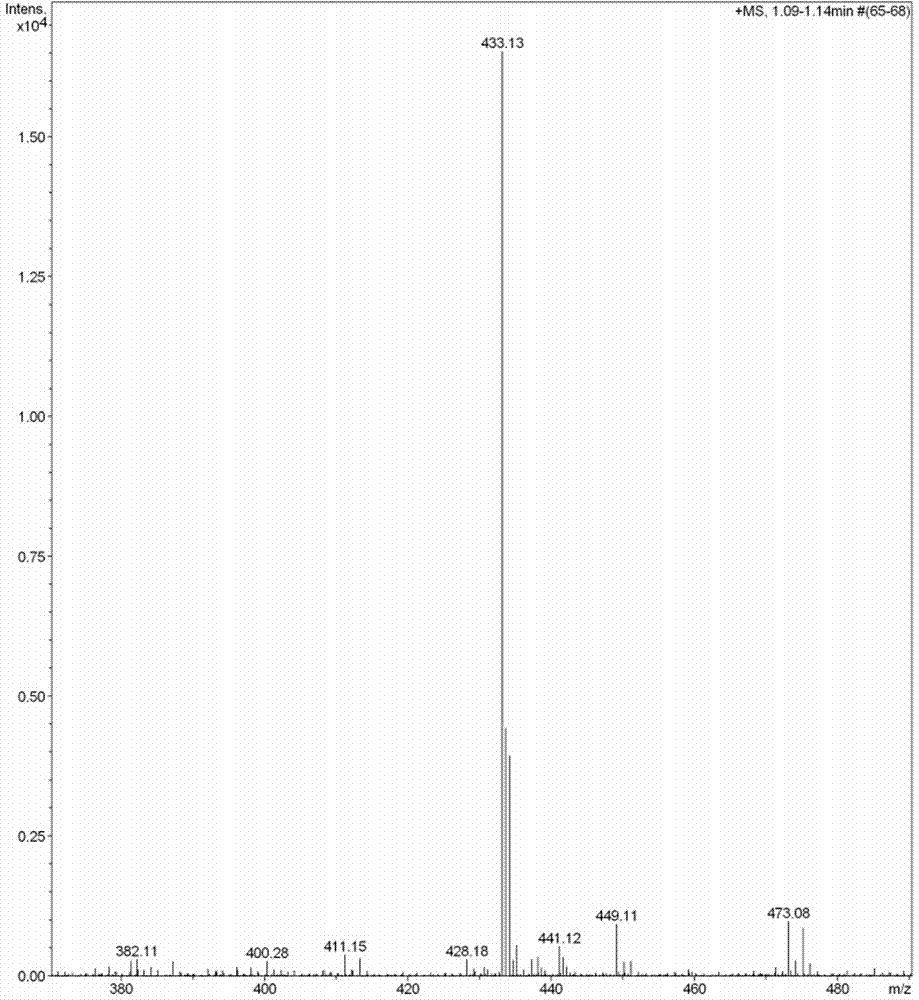
[0050] 2. Preparation of sulfi...
Embodiment 2
[0065] 1. Experimental part
[0066] Reagents and materials: All reagents were from Shanghai Jingchun Reagent Co., Ltd.
[0067] 1. Preparation of Intermediate I
[0068] In a 100mL round bottom flask, add 208mg (1mmol) 2-methylchromene malononitrile, 120mg (1mmol) p-hydroxybenzaldehyde, 20mL toluene, 1.0×10 -2 mmol piperidine, heated to reflux at 80°C for 24 hours, cooled, and removed the solvent with a rotary evaporator, the residue was extracted with dichloromethane and 2% sodium bicarbonate solution, and the organic layer was washed with anhydrous Na 2 SO 4 After drying, filtering, and concentration, the residue was passed through a silica gel column (PE:EtOAc=3:1) to obtain 128 mg of an orange-red solid with a yield of 41%.
[0069] 1 H-NMRδ (400MHz, DMSO-d 6 , ppm): 10.15 (s, 1H), 8.686-8.707 (d, J=8.4Hz, 1H), 7.871-7.910 (t, 1H), 7.742-7.763 (d, J=8.4Hz, 1H), 7.557- 7.673 (m, 4H), 7.211-7.251 (t, J=16Hz, 1H), 6.835-6.908 (t, J=8.0Hz, 3H).
[0070] 2. Preparation ...
Embodiment 3
[0085] 1. Experimental part
[0086] Reagents and materials: All reagents were from Shanghai Jingchun Reagent Co., Ltd.
[0087] 1. Preparation of Intermediate I
[0088] In a 100mL round bottom flask, add 208mg (1mmol) 2-methylchromene malononitrile, 180mg (1.5mmol) p-hydroxybenzaldehyde, 20mL n-propanol, 1.5×10 -2 mmol piperidine, heated to reflux at 100°C for 15 hours, cooled, removed the solvent with a rotary evaporator, extracted the residue with dichloromethane and 10% sodium bicarbonate solution, and washed the organic layer with anhydrous Na 2 SO 4 After drying, filtration and concentration, the residue was passed through a silica gel column (PE:EtOAc=3:1) to obtain 150 mg of an orange-red solid with a yield of 48%.
[0089] 1 H-NMRδ (400MHz, DMSO-d 6 , ppm): 10.15 (s, 1H), 8.686-8.707 (d, J=8.4Hz, 1H), 7.871-7.910 (t, 1H), 7.742-7.763 (d, J=8.4Hz, 1H), 7.557- 7.673 (m, 4H), 7.211-7.251 (t, J=16Hz, 1H), 6.835-6.908 (t, J=8.0Hz, 3H).
[0090] 2. Preparation of su...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com