Fluorescent compound and application thereof in hypochlorous acid detection
A technology of fluorescent compounds and hypochlorous acid, which is applied in the field of analysis and detection, can solve the problems of difficulty in realizing fluorescent test paper type detection, weakening of red fluorescence, and increase of blue fluorescence intensity, etc., and achieves high accuracy, easy aggregation, and simple preparation process. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
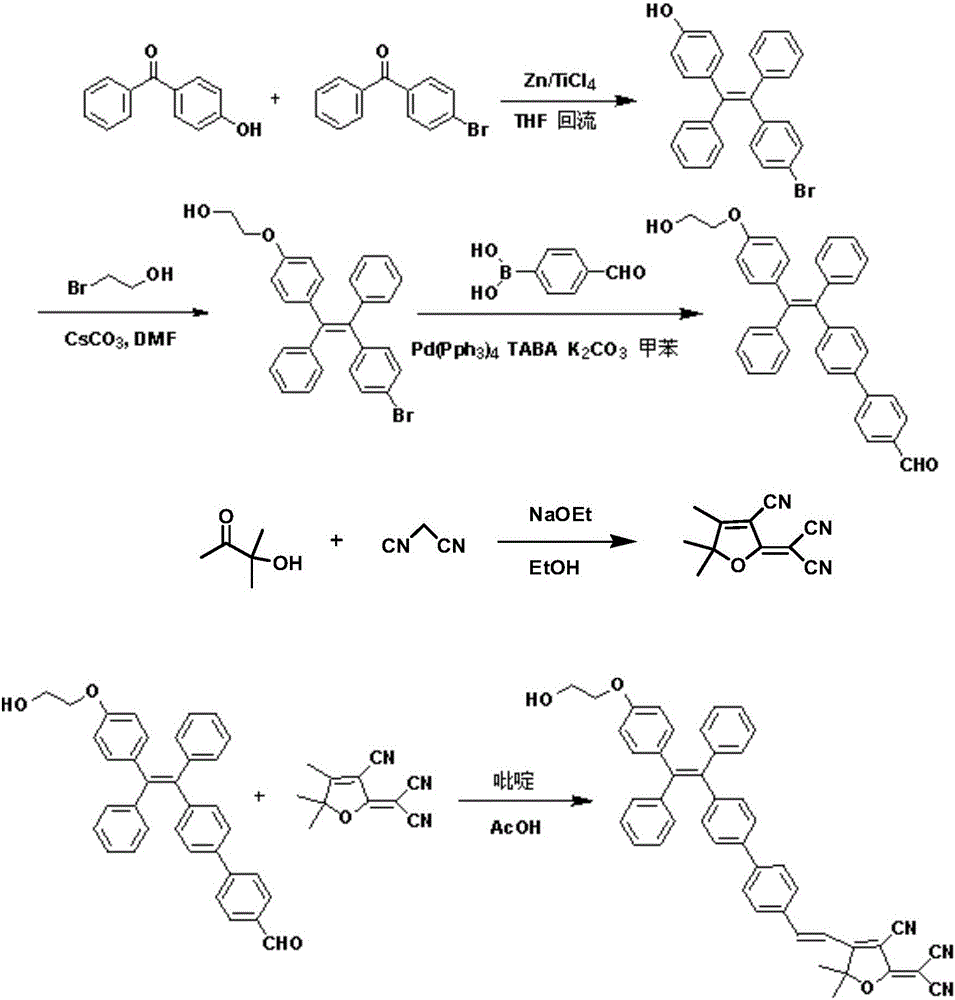
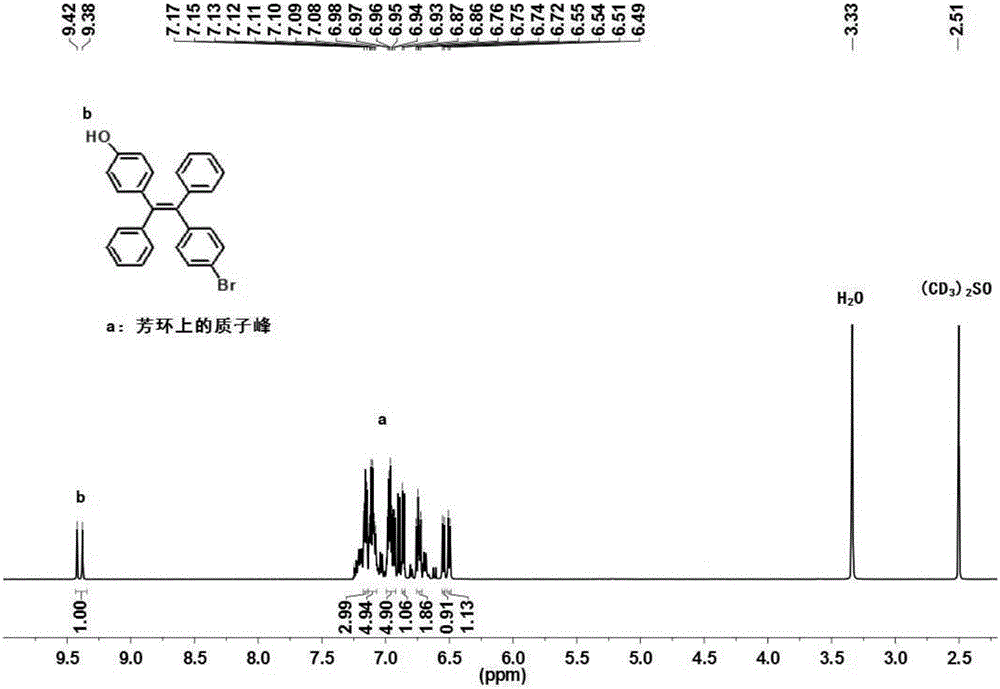
[0047] (1) 1982mg (4-hydroxy-phenyl)-phenyl-methanone (10mmol) and 2611mg (4-bromophenyl)-phenyl-methanone (10mmol) were dissolved in 120mL tetrahydrofuran, and 3923mg zinc was added under stirring Powder (60mmol), nitrogen protection, 7.40mL titanium tetrachloride (67.5mmol) was added dropwise under ice bath conditions, the ice bath was removed, first reacted at room temperature for 1 hour, then heated to reflux overnight, and the temperature was controlled at 70°C; After cooling to room temperature, it was extracted with ethyl acetate / saturated sodium bicarbonate solution, and the organic phase was dried over anhydrous sodium sulfate and filtered; the organic solvent was removed by rotary evaporation, and the resulting solid was purified by silica gel chromatography (eluent: n-hexane / dichloromethane, V / V=1:3), to obtain white solid product 4-[2-(4-bromo-phenyl)-1,2-diphenyl-vinyl]-phenol 1800mg (yield is 42.1%); the product was characterized by 1H NMR, 1H NMR (600MHz, DMSO,...
Embodiment 2
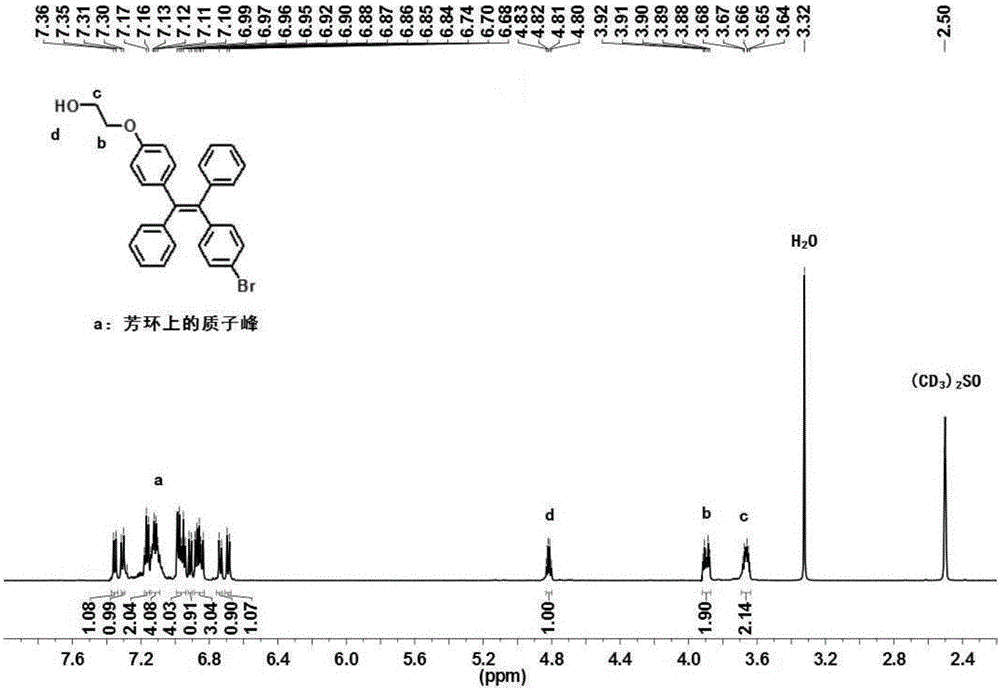
[0053] (1) 932mg (4-hydroxy-phenyl)-phenyl-methanone (4.70mmol) and 1364mg (4-bromophenyl)-phenyl-methanone (5.22mmol) were dissolved in 47mL tetrahydrofuran and added under stirring 1690mg of zinc powder (25.85mmol), under nitrogen protection, 2.83mL of titanium tetrachloride (25.85mmol) was added dropwise under ice bath conditions, removed from the ice bath, first reacted at room temperature for 0.5 hours, then heated and refluxed overnight, and controlled the temperature to 67°C; after cooling to room temperature, extract with ethyl acetate / saturated sodium bicarbonate solution, dry the organic phase with anhydrous sodium sulfate, and filter; remove the organic solvent by rotary evaporation, and the resulting solid is purified by silica gel chromatography (eluent : n-hexane / dichloromethane, V / V=1:3), to obtain white solid product 4-[2-(4-bromo-phenyl)-1,2-diphenyl-vinyl]-phenol 837mg ( Yield 41.7%).
[0054] (2) Dissolve 641mg 4-[2-(4-bromo-phenyl)-1,2-diphenyl-vinyl]-phen...
Embodiment 3
[0060] (1) 595mg (4-hydroxy-phenyl)-phenyl-methanone (3mmol) and 825mg (4-bromophenyl)-phenyl-methanone (3.16mmol) were dissolved in 34.5mL tetrahydrofuran and added under stirring 1118mg of zinc powder (17.1mmol), protected by nitrogen gas, added 2.09mL of titanium tetrachloride (19.07mmol) dropwise under ice bath conditions, removed the ice bath, first reacted at room temperature for 0.7 hours, then heated to reflux overnight, and controlled the temperature to 68°C; after cooling to room temperature, extract with ethyl acetate / saturated sodium bicarbonate solution, dry the organic phase with anhydrous sodium sulfate, filter; remove the organic solvent by rotary evaporation, and purify the obtained solid by silica gel chromatography (eluent : n-hexane / methylene chloride, V / V=1:3), to obtain white solid product 4-[2-(4-bromo-phenyl)-1,2-diphenyl-vinyl]-phenol 537mg ( Yield 41.9%).
[0061] (2) Dissolve 427mg 4-[2-(4-bromo-phenyl)-1,2-diphenyl-vinyl]-phenol (1mmol) in 6.5mL N,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com