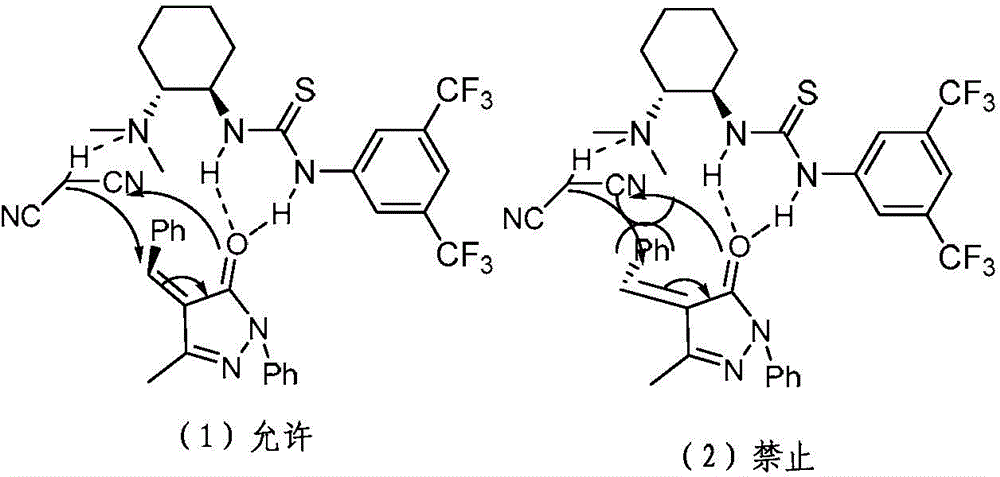
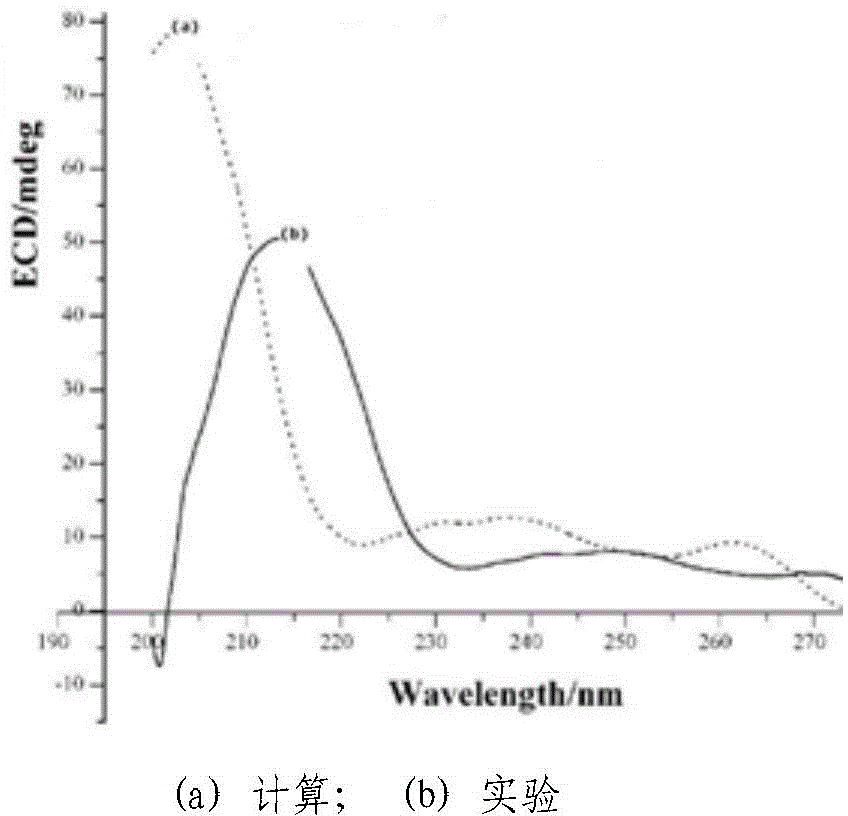
Chiral 1, 4-dihydropyran (2, 3-c) pyrazole derivative as well as synthesis method and application of chiral 1, 4-dihydropyran (2, 3-c) pyrazole derivative
A technology of pyrazole derivatives and dihydropyran, which is applied in the direction of organic active ingredients, chemicals used for biological control, medical preparations containing active ingredients, etc., can solve the problems that there are no literature reports on pyrazole derivatives, etc. Achieving the effects of flexible reaction time, wide application range and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
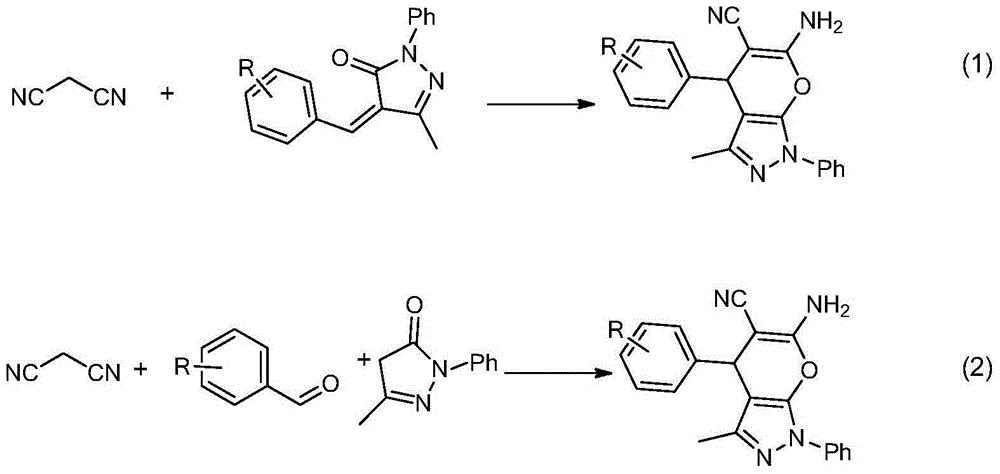
[0039] Add pyrazolone derivative 2a (0.10mmol), malononitrile (0.10mmol) and Takemoto catalyst (0.02mmol) as shown in formula (IV) to a 5mL reaction test tube, then add 1ml of toluene, and the mixture is heated at a temperature of 15 °C and stirred for 12 hours. Then the solvent was removed under reduced pressure, and column chromatography was performed with a mixture of petroleum ether and ethyl acetate (the volume ratio of petroleum ether and ethyl acetate was 10:1) as the eluent to obtain chiral 1,4- Dihydropyran(2,3-c)pyrazole derivative 3a (31 mg, 95% yield).
[0040] The reaction equation is as follows:
[0041]
[0042] The structure of the chiral 1,4-dihydropyran(2,3-c)pyrazole derivative 3a obtained above was identified by nuclear magnetic resonance and high-resolution mass spectrometry, 1 H NMR(400MHz,DMSO)δ(ppm)7.79(d,J=8.1Hz,2H),7.49(t,J=7.9Hz,2H),7.37-7.22(m,8H),4.67(s,1H) ,1.77(s,3H); 13 C NMR (101MHz, CDCl 3)δ (ppm) 159.50, 145.36, 143.94, 143.67, 137.60...
Embodiment 2
[0046] Add pyrazolone derivative 2b (0.10mmol), malononitrile (0.10mmol) and Takemoto catalyst (0.02mmol) as shown in formula (IV) to a 5mL reaction test tube, then add 1ml of toluene, and the mixture is heated at a temperature of 15 °C and stirred for 12 hours. Then the solvent was removed under reduced pressure, and column chromatography was carried out with a mixture of petroleum ether and ethyl acetate (the volume ratio of petroleum ether and ethyl acetate was 10:1) as the eluent to obtain the chiral 1,4- Dihydropyran(2,3-c)pyrazole derivative 3b (32 mg, yield 93%).
[0047] The reaction equation is as follows:
[0048]
[0049] The structure of the chiral 5-chloro-1,4-dihydropyran(2,3-c)pyrazole derivative 3b obtained above was identified by nuclear magnetic resonance and high-resolution mass spectrometry, 1 H NMR(600MHz,DMSO)δ(ppm)7.78(d,J=7.8Hz,2H),7.49(t,J=7.9Hz,2H),7.33-7.25(m,5H),7.17(t,J= 8.8Hz,2H),4.72(s,1H),1.78(s,3H); 13 C NMR (151MHz, DMSO) δ (ppm) 162.02...
Embodiment 3
[0052] Add pyrazolone derivative 2c (0.10mmol), malononitrile (0.10mmol) and Takemoto catalyst (0.02mmol) as shown in formula (IV) to a 5mL reaction test tube, then add 1ml of toluene, and the mixture is heated at a temperature of 15 °C and stirred for 12 hours. Then the solvent was removed under reduced pressure, and column chromatography was performed with a mixture of petroleum ether and ethyl acetate (the volume ratio of petroleum ether and ethyl acetate was 10:1) as the eluent to obtain chiral 1,4- Dihydropyran(2,3-c) pyrazole derivative 3c (35 mg, 97% yield).
[0053] The reaction equation is as follows:
[0054]
[0055] The structure of the 5-chloro-1,4-dihydropyran(2,3-c)pyrazole derivative 3c obtained above was identified by NMR and high-resolution mass spectrometry, 1 H NMR(600MHz,DMSO)δ(ppm)7.78(d,J=7.7Hz,2H),7.49(t,J=7.9Hz,2H),7.41(d,J=8.4Hz,2H),7.30(t ,J=8.0Hz,5H),4.72(s,1H),1.78(s,3H); 13 C NMR (151MHz, DMSO) δ (ppm) 159.53, 145.26, 143.96, 142.73, 137.54...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com