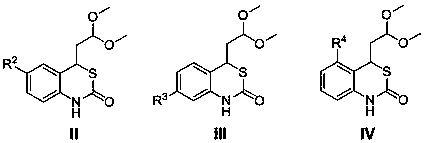
1,3-benzothiazin-2-one derivatives having antibacterial activity, and synthetic method and applications thereof
A benzothiazine and antibacterial activity technology, applied in the field of 1,3-benzothiazine-2-one derivatives and their synthesis, can solve the problems of complex substrates, harsh reaction conditions, etc. Easy to operate, flexible response time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] 65 o Under C, add the substituted α,β-unsaturated aldehyde derivative 1a (189 mg, 1 mmol ), 4 ml of methanol into a 10 ml reaction flask, and then add hydroxylamine hydrochloride 2 (210 mg, 3 mmol), The reaction was stirred for 24 hours. Then the solvent was removed under reduced pressure, and the eluent was subjected to column chromatography with a mixture of petroleum ether and ethyl acetate (the volume ratio of petroleum ether and ethyl acetate was 5:1) to obtain 1,3-benzothiophene with the structural formula 3a Oxin-2-one derivative 3a (106 mg, yield 42%). The reaction equation is as follows:
[0037]
[0038] The structure of the 1,3-benzothiazin-2-one derivative 3a obtained above was identified by nuclear magnetic resonance and high-resolution mass spectrometry, 1 H NMR (600 MHz, CDCl 3 ) δ 9.93 (s, 1H), 7.26 (td, J = 7.8, 1.3 Hz, 1H),7.16 (d, J = 6.7 Hz, 1H), 7.09 (td, J = 7.5, 0.9 Hz, 1H), 7.02 (d, J = 7.9Hz, 1H), 4.45 (t, J = 5.9 Hz, 1H), 4.16 ...
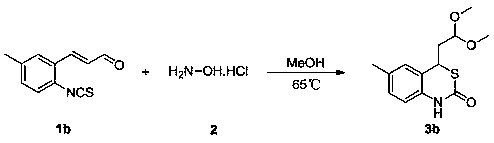
Embodiment 2
[0040] 65 o C, the substituted α,β-unsaturated aldehyde derivative 1b (203 mg, 1 mmol, ), 4 ml of methanol were sequentially added to a 10 ml reaction flask, followed by addition of hydroxylamine hydrochloride 2 (210 mg, 3 mmol) , and the reaction was stirred for 24 hours. Then the solvent was removed under reduced pressure, and the eluent was subjected to column chromatography with a mixture of petroleum ether and ethyl acetate (the volume ratio of petroleum ether and ethyl acetate was 5:1) to obtain 1,3-benzothiophene with the structural formula 3b Oxin-2-one derivative 3b (96 mg, yield 36%). The reaction equation is as follows:
[0041]
[0042] The structure of the 1,3-benzothiazin-2-one derivative 3b obtained above was identified by nuclear magnetic resonance and high-resolution mass spectrometry, 1 H NMR (600 MHz, CDCl 3 ) δ 9.65 (s, 1H), 7.06 (dd, J = 8.1, 1.2 Hz, 1H), 6.96 (s, 1H), 6.89 (d, J = 8.1 Hz, 1H), 4.47 (dd, J = 6.1, 5.7 Hz, 1H), 4.10(dd, J = 8....
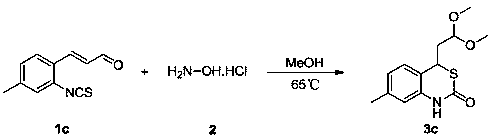
Embodiment 3
[0044] 65 o C, the substituted α,β-unsaturated aldehyde derivative 1c (203 mg, 1 mmol, ), 4 ml of methanol were sequentially added to a 10 ml reaction flask, followed by addition of hydroxylamine hydrochloride 2 (210 mg, 3 mmol) , and the reaction was stirred for 24 hours. Then the solvent was removed under reduced pressure, and the eluent was subjected to column chromatography with a mixture of petroleum ether and ethyl acetate (the volume ratio of petroleum ether and ethyl acetate was 5:1) to obtain 1,3-benzothiophene with the structural formula 3c Oxin-2-one derivative 3c (91 mg, yield 34%). The reaction equation is as follows:
[0045]
[0046] The structure of the 1,3-benzothiazin-2-one derivative 3c obtained above was identified by nuclear magnetic resonance and high-resolution mass spectrometry, 1 H NMR (600 MHz, CDCl 3 ) δ 9.68 (s, 1H), 7.03 (d, J = 7.7 Hz, 1H), 6.92 –6.89 (m, 1H), 6.81 (s, 1H), 4.44 (t, J = 5.9 Hz, 1H), 4.12 (t, J = 7.8 Hz,1H), 3.33 (d, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com