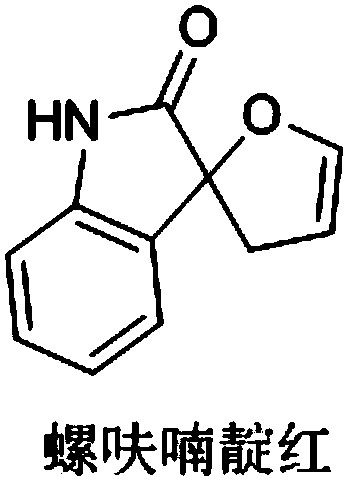
A kind of double spiro isatin furan derivative with antibacterial activity and its synthesis method and application
A technology of furan derivatives and synthesis methods, applied in the field of bispiroisatin furan derivatives and their synthesis, can solve the problems of antibacterial properties of compounds that have not been studied, and achieve the effects of flexible reaction time, high yield, and wide application range
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] In a 5 ml reaction tube, add 1 ml of tetrahydrofuran, substituted 3-hydroxyisatin 1a (36 mg, 0.15 mmol, 1.5 eq), electron-deficient olefin 2a (20 mg, 0.1 mmol, 1.0 eq) and cinchonine (6 mg, 0.02 mmol ) was stirred at 0° C. for 12 h. The solvent is removed under reduced pressure subsequently, and the eluent is carried out column chromatography with the mixed solution of petroleum ether and ethyl acetate (the volume ratio of petroleum ether and ethyl acetate is 8:1) to obtain the double spiro isatin furan derivative of structural formula such as 3aa 3aa (31 mg, 71% yield). The reaction equation is as follows:
[0044]
[0045] The structure of the bis-helical isatin furan derivative 3aa obtained above was identified by nuclear magnetic resonance and high-resolution mass spectrometry, 1 H NMR (400MHz, DMSO) δ10.44(s,1H),7.99(s,2H),7.62(d,J=7.6Hz,1H),7.38(d,J=7.6Hz,1H),7.31–7.20 (m,2H),7.17(dd,J=10.8,7.2Hz,3H),7.05(s,1H),6.94(s,1H),6.80–6.70(m,3H),6.60(d,J=7.9 Hz,1H)...
Embodiment 2
[0047] In a 5 ml reaction tube, add 1 ml of tetrahydrofuran, substituted 3-hydroxyisatin 1a (36 mg, 0.15 mmol, 1.5 eq), electron-deficient olefin 2b (25 mg, 0.1 mmol, 1.0 eq) and cinchonine (6 mg, 0.02 mmol ) was stirred at 0° C. for 12 h. Subsequently, the solvent is removed under reduced pressure, and column chromatography is carried out with a mixed solution of sherwood oil and ethyl acetate (the volume ratio of sherwood oil and ethyl acetate is 8:1) as eluent to obtain a double-spirited isatin furan derivative of structural formula such as 3ab 3ab (25 mg, 51% yield). The reaction equation is as follows:
[0048]
[0049] The structure of the bis-helical isatin furan derivative 3ab obtained above was identified by nuclear magnetic resonance and high-resolution mass spectrometry, 1H NMR (600MHz, DMSO) δ7.98 (s, 2H), 7.53 (d, J = 7.6Hz, 1H), 7.39 (d, J = 7.5Hz, 1H), 7.36–7.31 (m, 1H), 7.24 (td, J=7.8,1.0Hz,1H),7.18(d,J=7.2Hz,1H),7.15(t,J=7.3Hz,2H),7.01(dt,J=20.9,7.6Hz,2...
Embodiment 3
[0051] In a 5 ml reaction tube, add 1 ml of tetrahydrofuran, substituted 3-hydroxyisatin 1a (36 mg, 0.15 mmol, 1.5 eq), electron-deficient olefin 2c (29 mg, 0.1 mmol, 1.0 eq) and cinchonine (6 mg, 0.02 mmol ) was stirred at 0° C. for 12 h. Subsequently, the solvent is removed under reduced pressure, and the eluent is carried out by column chromatography with a mixture of petroleum ether and ethyl acetate (the volume ratio of petroleum ether and ethyl acetate is 8:1) to obtain a double-spirited isatin furan derivative of structural formula such as 3ac 3ac (31 mg, 58% yield). The reaction equation is as follows:
[0052]
[0053] The structure of the double-helical isatin furan derivative 3ac obtained above was identified by nuclear magnetic resonance and high-resolution mass spectrometry, 1 H NMR (600MHz, DMSO) δ8.05(s, 2H), 7.56(d, J=7.6Hz, 1H), 7.42(d, J=7.5Hz, 1H), 7.31(td, J=7.8, 1.2Hz ,1H),7.22(td,J=7.8,1.2Hz,1H),7.16–7.10(m,3H),7.08(d,J=7.7Hz,1H),7.07–7.03(m,2H),6.9...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com