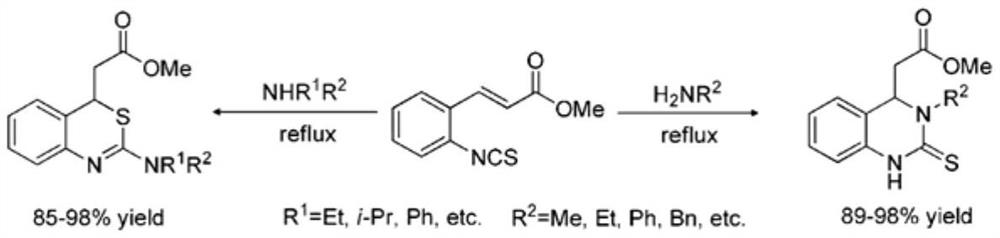
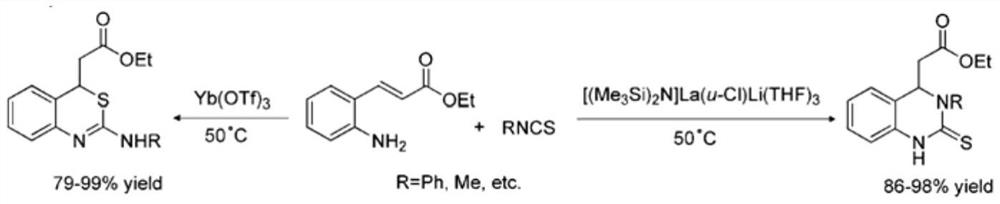
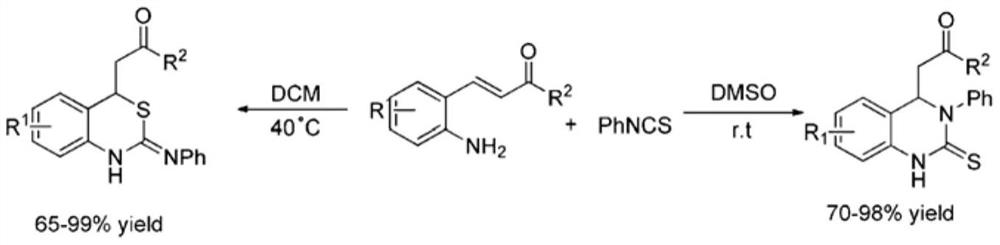
A kind of 4h-1,3-benzothiazine derivative with antibacterial activity and its synthesis method and application
A 4H-1, benzothiazine technology, applied in the directions of botanical equipment and methods, applications, biocides, etc., can solve the problems of complex substrates, harsh reaction conditions, low yields, etc., and achieves high yields, Easy to operate, cheap and easy to obtain solvent
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0049] At 25°C, substituted cinnamaldehyde derivative 1b (26.7mg, 0.1mmol), and 1ml of methanol were sequentially added to a 5ml reaction flask, followed by secondary amine 2a (11.0mg, 0.15mmol), and the reaction was stirred for 30 Second. Then the solvent was removed under reduced pressure, and the mixed solution of petroleum ether and ethyl acetate (the volume ratio of petroleum ether and ethyl acetate was 8:1) was used as eluent to carry out column chromatography to obtain 4H-1,3-benzene of structural formula such as 3ba. Thiazine derivative 3ba (33 mg, yield 98%). The reaction equation is as follows:
[0050]
[0051] The structure of the 4H-1,3-benzothiazine derivative 3ba obtained above was identified by nuclear magnetic resonance and high-resolution mass spectrometry, 1 H NMR (600MHz, CDCl 3 )δ9.73(d,J=1.5Hz,1H),7.21(d,J=7.6Hz,1H),7.11–7.01(m,2H),5.02(dd,J=10.4,3.6Hz,1H), 3.61(d, J=91.2Hz, 4H), 2.95(ddd, J=17.4, 10.4, 2.2Hz, 1H), 2.68(dd, J=17.4, 3.6Hz, 1H), 1.96...
Embodiment 2
[0053] At 25°C, substituted cinnamaldehyde derivative 1c (18.9mg, 0.1mmol), and 1ml of methanol were sequentially added to a 5ml reaction flask, followed by secondary amine 2a (11.0mg, 0.15mmol), and the reaction was stirred for 30 Second. Then remove the solvent under reduced pressure, and carry out column chromatography with a mixture of petroleum ether and ethyl acetate (the volume ratio of petroleum ether and ethyl acetate is 8:1) as the eluent to obtain 4H-1,3-benzene with a structural formula such as 3ca Thiazine derivative 3ca (33 mg, yield 93%). The reaction equation is as follows:
[0054]
[0055] The structure of the 4H-1,3-benzothiazine derivative 3ca obtained above was identified by nuclear magnetic resonance and high-resolution mass spectrometry, 1 H NMR (600MHz, CDCl 3 )δ9.66(s,1H),7.31(dd,J=8.5,2.3Hz,1H),7.20(d,J=2.3Hz,1H),6.97(d,J=8.5Hz,1H),4.45( dd,J=8.3,5.8Hz,1H),3.66(s,2H),3.53(s,2H),2.99(ddd,J=17.9,8.4,1.5Hz,1H),2.82(dd,J=17.8, 5.8Hz,1H),1.97–1.91(...
Embodiment 3
[0057] At 25°C, substituted cinnamaldehyde derivative 1d (20.3mg, 0.1mmol), and 1ml of methanol were sequentially added to a 5ml reaction flask, followed by secondary amine 2a (11.0mg, 0.15mmol), and the reaction was stirred for 30 Second. Subsequently, the solvent was removed under reduced pressure, and column chromatography was carried out using a mixture of petroleum ether and ethyl acetate (the volume ratio of petroleum ether and ethyl acetate was 8:1) as the eluent to obtain 4H-1,3-benzene with a structural formula such as 3da. Thiazine derivative 3da (26 mg, yield 95%). The reaction equation is as follows:
[0058]
[0059] The structure of the 4H-1,3-benzothiazine derivative 3da obtained above was identified by nuclear magnetic resonance and high-resolution mass spectrometry, 1 H NMR (600MHz, CDCl 3 )δ9.68(s,1H),7.06–7.00(m,2H),6.87(s,1H),4.44(dd,J=8.4,5.8Hz,1H),3.71–3.64(m,2H),3.54 (s,2H),3.00(ddd,J=17.6,8.5,1.7Hz,1H),2.82(ddd,J=17.7,5.8,0.8Hz,1H),2.29(s,3H),1.9...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com