Method for preparing 2-chloronicotinic acid
A technology of chloronicotinic acid and methyl formate, applied in the direction of organic chemistry, etc., can solve the problems of difficult to obtain raw materials, harsh requirements, and large investment in equipment, and achieve the effect of easy-to-obtain raw materials, mild and efficient reaction conditions, and low price
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
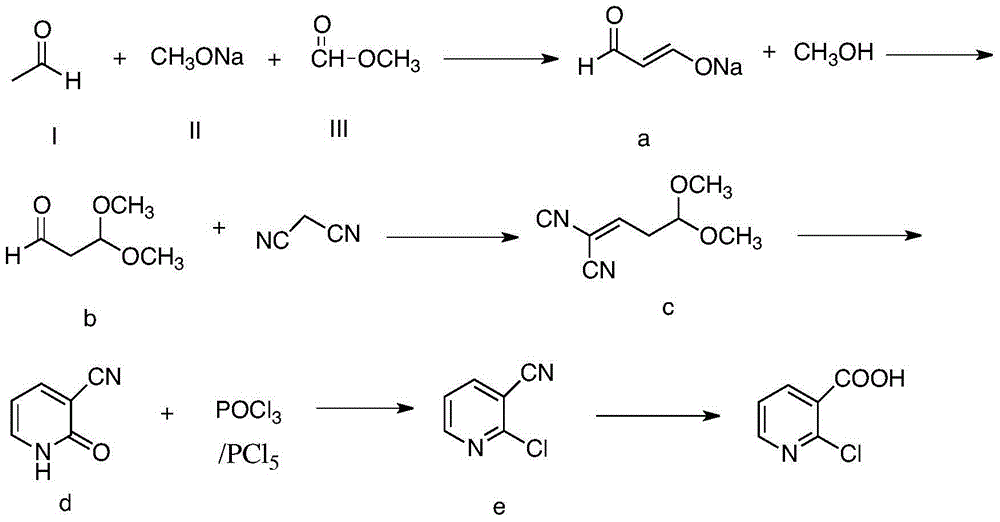
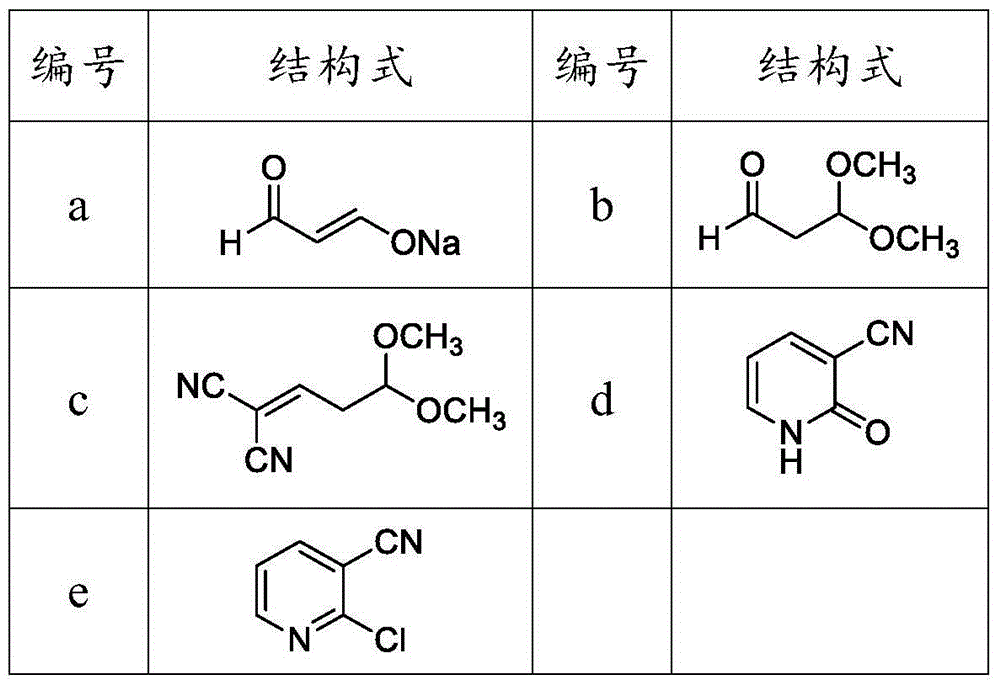
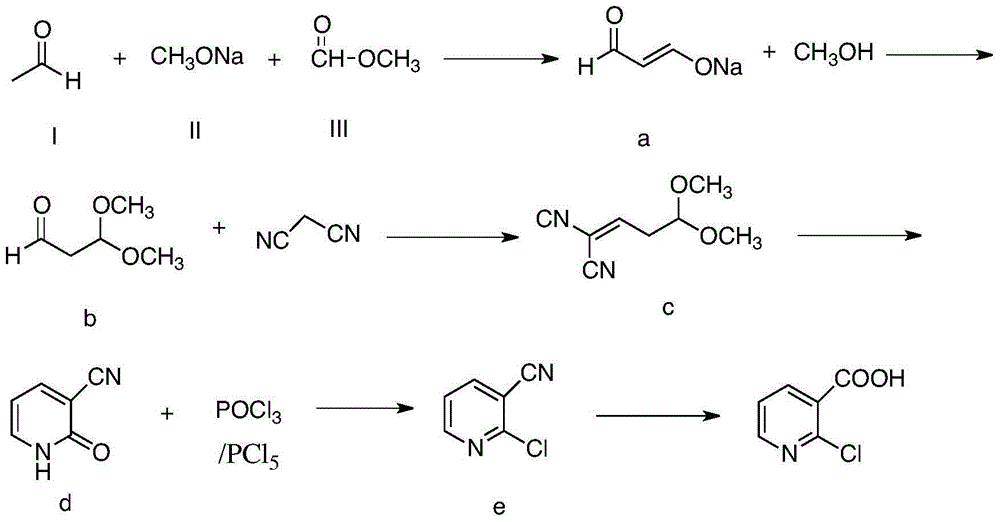
[0029] (1) Add compound (I) (44g, 1.0mol), compound (II) (85g, 1.0mol) into a 250mL four-neck flask, slowly add compound (III) (60g, 1.0mol) dropwise, and control the temperature to low At 25°C, after the dropwise addition, the temperature was raised to 40°C for 2 hours to obtain compound a (the reaction product of this step was detected by gas chromatography, the purity was 85%, and the yield was 90%, calculated as acetaldehyde).
[0030] (2) Add 100g of methanol into a 500mL four-necked bottle, and slowly add compound a (189g) and sulfuric acid dropwise at the same time. After 1 hour, the salt was removed by suction filtration, and 111 g of compound b was obtained after purification by vacuum distillation (the reaction product of this step was detected by gas chromatography, the purity was 88%, and the yield was 92%).
[0031] (3) Compound b (111 g), toluene (400 g), malononitrile (66 g, 1.0 mol), and ammonium acetate (2 g) were added into a 1000 mL four-necked flask, and ke...
Embodiment 2
[0036] (1) Add compound (I) (44g, 1.0mol), compound (II) (127.5g, 1.5mol) into a 250mL four-neck flask, slowly add compound (III) (90g, 1.5mol) dropwise, and control the temperature Lower than 10°C, after the dropwise addition, the temperature was raised to 0°C for 2 hours to obtain 189g of compound a (the reaction product of this step was detected by gas chromatography, the purity was 87%, and the yield was 45%, calculated as acetaldehyde).
[0037](2) Add 100g of methanol into a 500mL four-necked bottle, and slowly add compound a (189g) and sulfuric acid dropwise at the same time, control the temperature below 20°C, and the pH value below 2. After 1 hour, the salt was removed by suction filtration, and 47 g of compound b was obtained after purification by vacuum distillation (the reaction product of this step was detected by gas chromatography, the purity was 88%, and the yield was 78%).
[0038] (3) Compound b (47g), toluene (400g), malononitrile (66g, 1.0mol) and ammonium ...
Embodiment 3
[0043] (1) Add compound (I) (44g, 1.0mol), compound (II) (425g, 5.0mol) into a 250mL four-neck flask, slowly add compound (III) (300g, 5.0mol) dropwise, and control the temperature to low At 25°C, after the dropwise addition, the temperature was raised to 80°C for 2 hours to obtain 189g of compound a (the reaction product of this step was detected by gas chromatography, the purity was 83%, and the yield was 79%, calculated as acetaldehyde).
[0044] (2) Add 100g of methanol into a 500mL four-necked bottle, and slowly add compound a (189g) and sulfuric acid dropwise at the same time. After 1 hour, the salt was removed by suction filtration, and 86 g of compound b was obtained after purification by vacuum distillation (the reaction product of this step was detected by gas chromatography, the purity was 88%, and the yield was 82%).
[0045] (3) Compound b (86g), toluene (400g), malononitrile (66g, 1.0mol) and ammonium acetate (2g) were added into a 1000mL four-necked flask, and k...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com