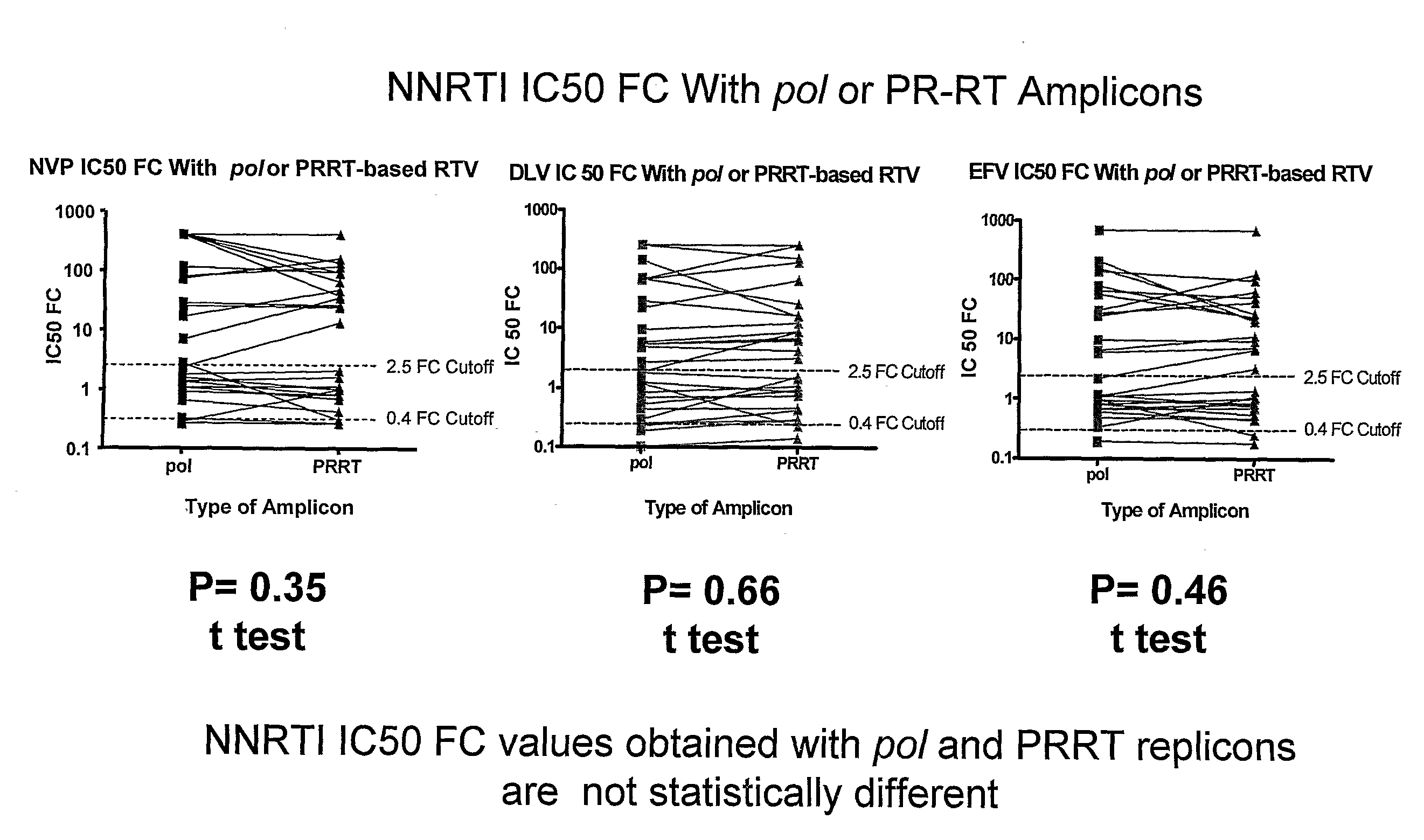
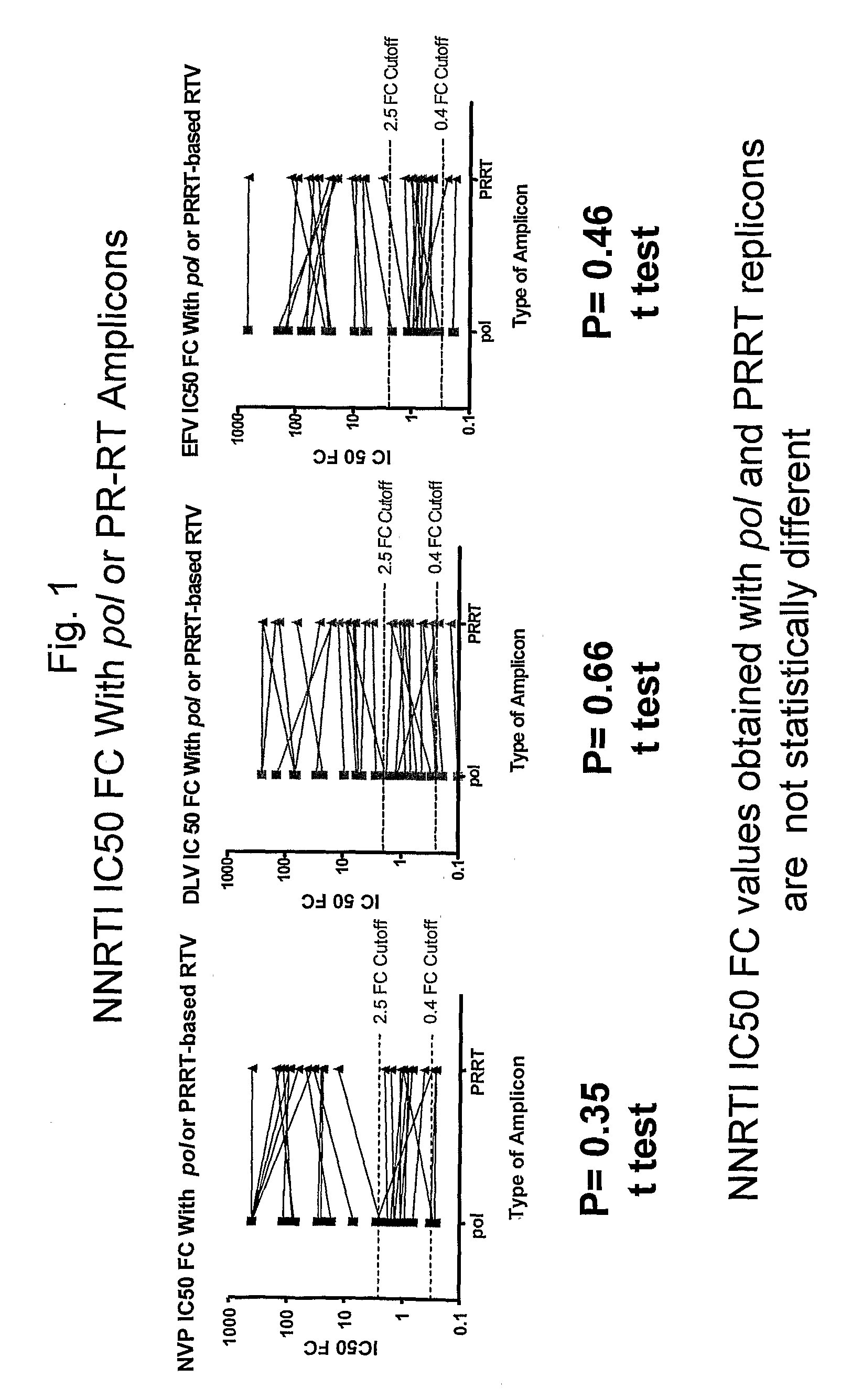
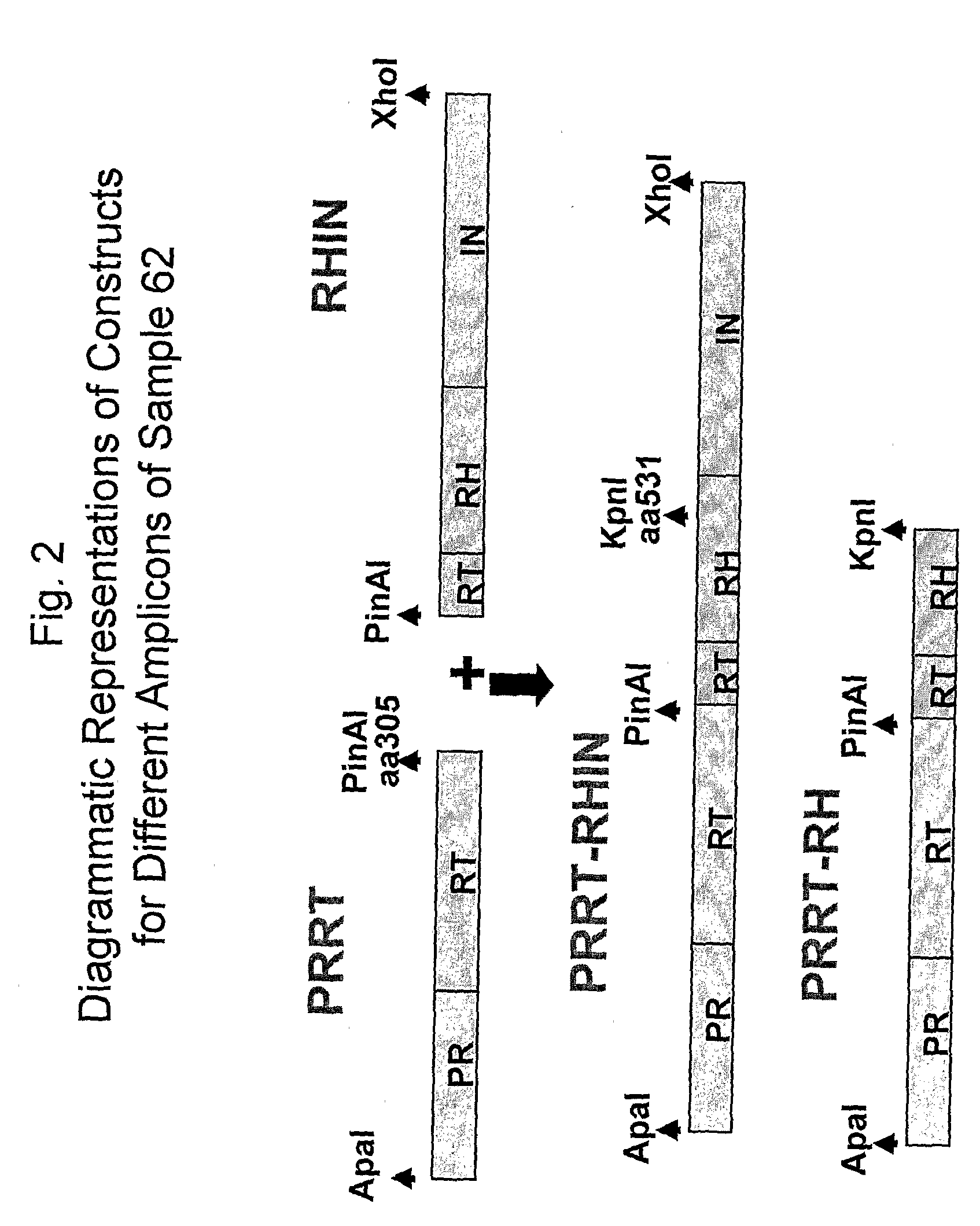
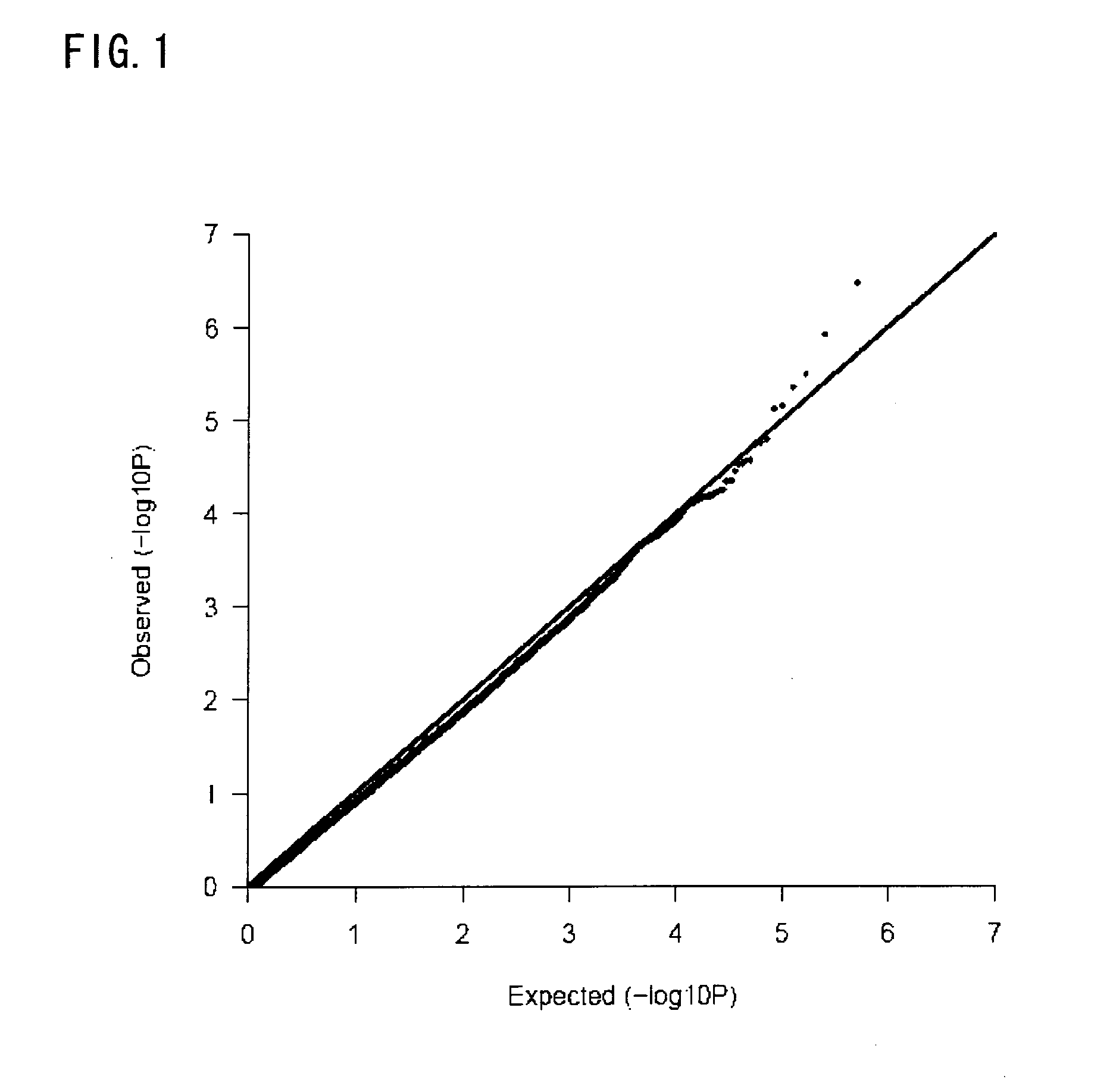
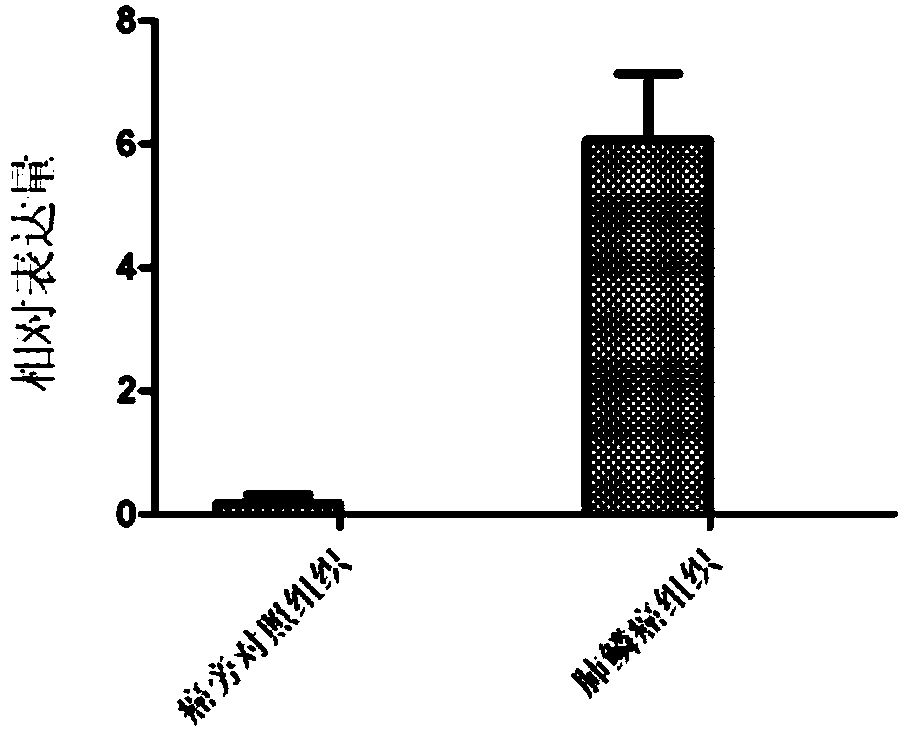Patents
Literature
63 results about "Nevirapine" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Nevirapine is used with other HIV medications to help control HIV infection.
Pharmaceutical antiretroviral composition
InactiveUS20140193491A1Easy to manufactureBiocideOrganic active ingredientsNucleoside Reverse Transcriptase InhibitorEmtricitabine
The present invention relates to a pharmaceutical antiretroviral composition comprising (i) a nucleoside reverse-transcriptase inhibitor selected from lamivudine and emtricitabine, (ii) extended release nevirapine, and (iii) tenofovir; a process for preparing such composition and the use of such composition in medicine, particularly for the prophylaxis and / or treatment of diseases caused by retroviruses.
Owner:CIPLA LTD
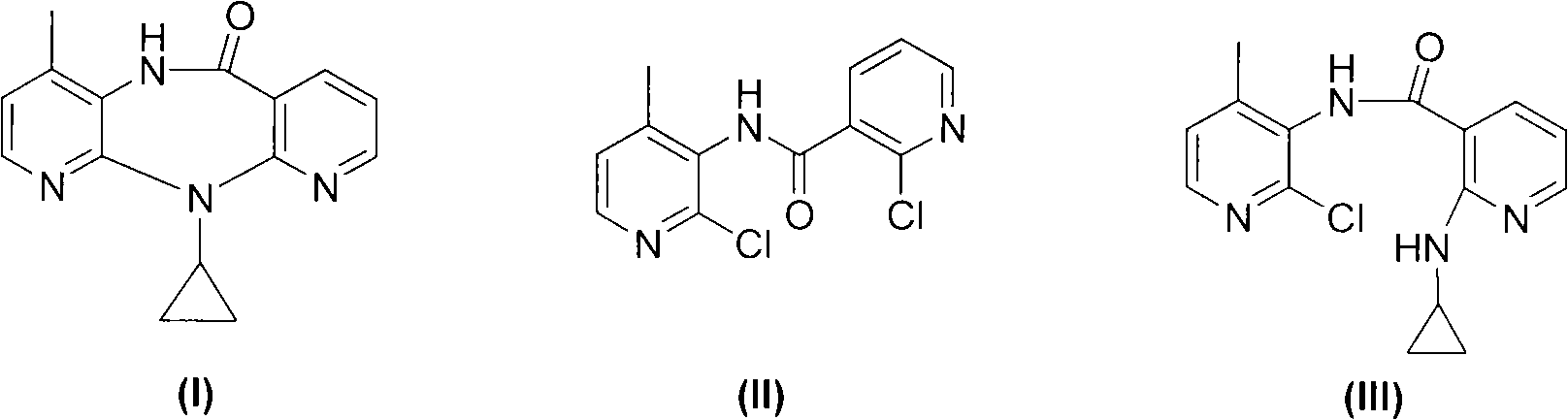
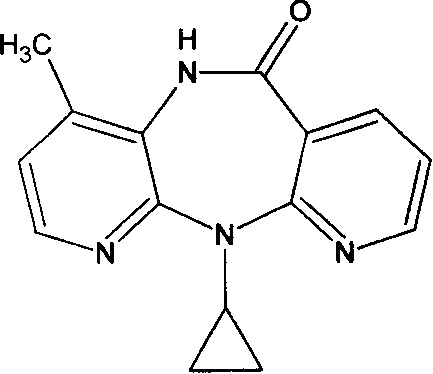
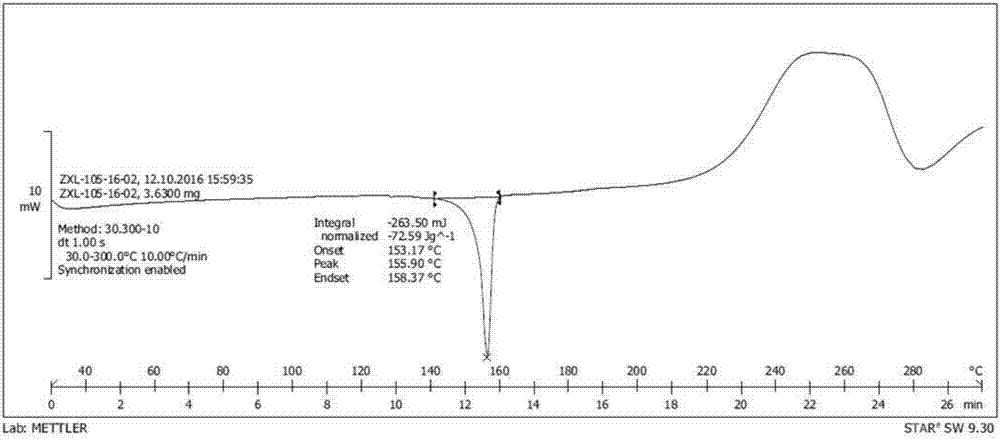
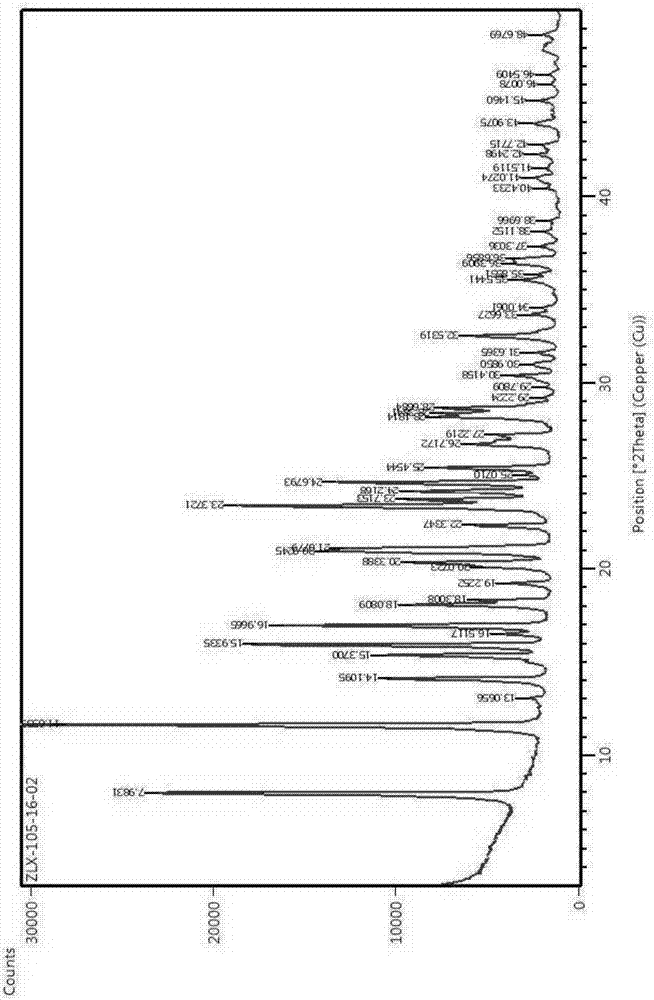
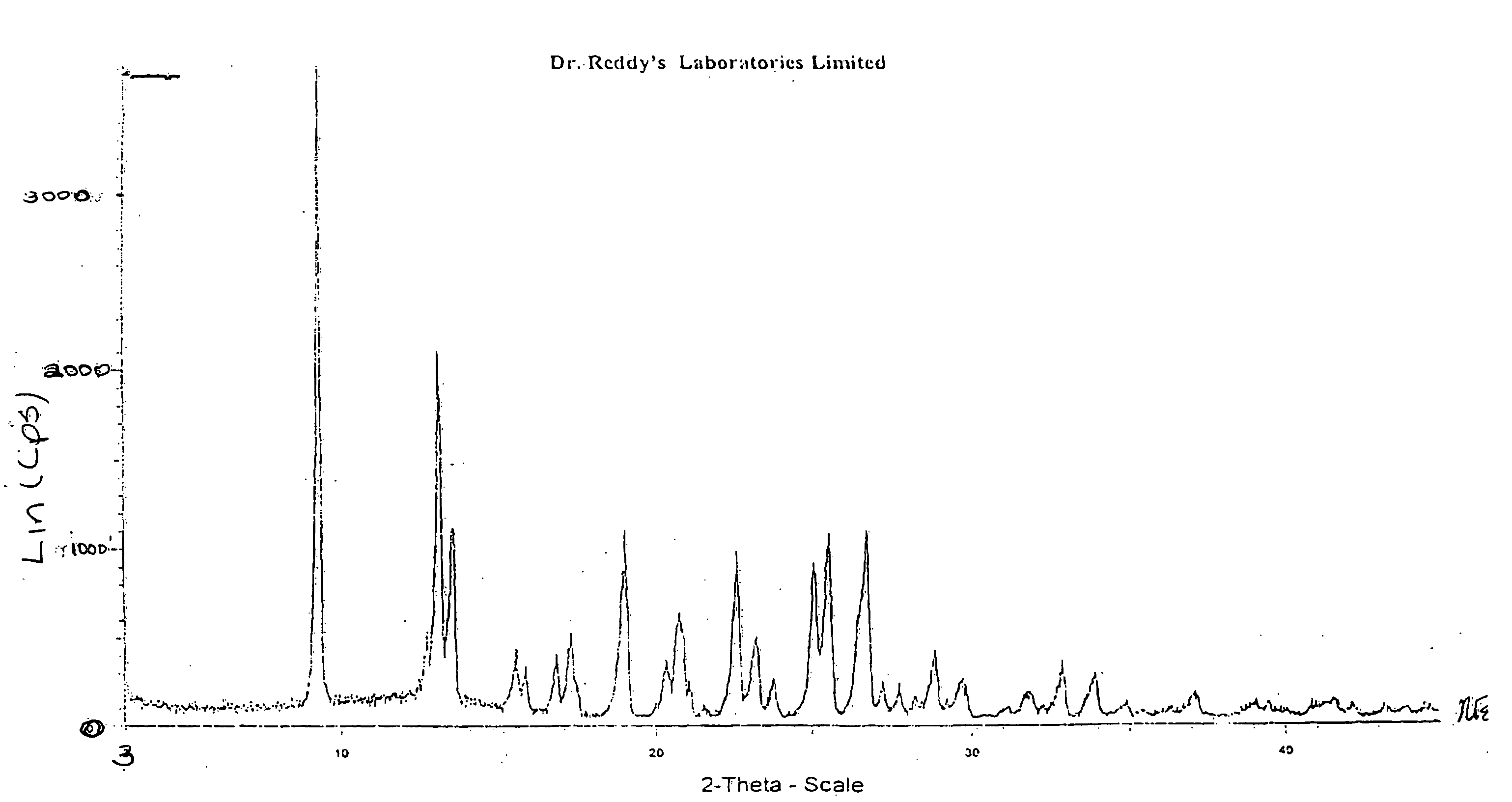
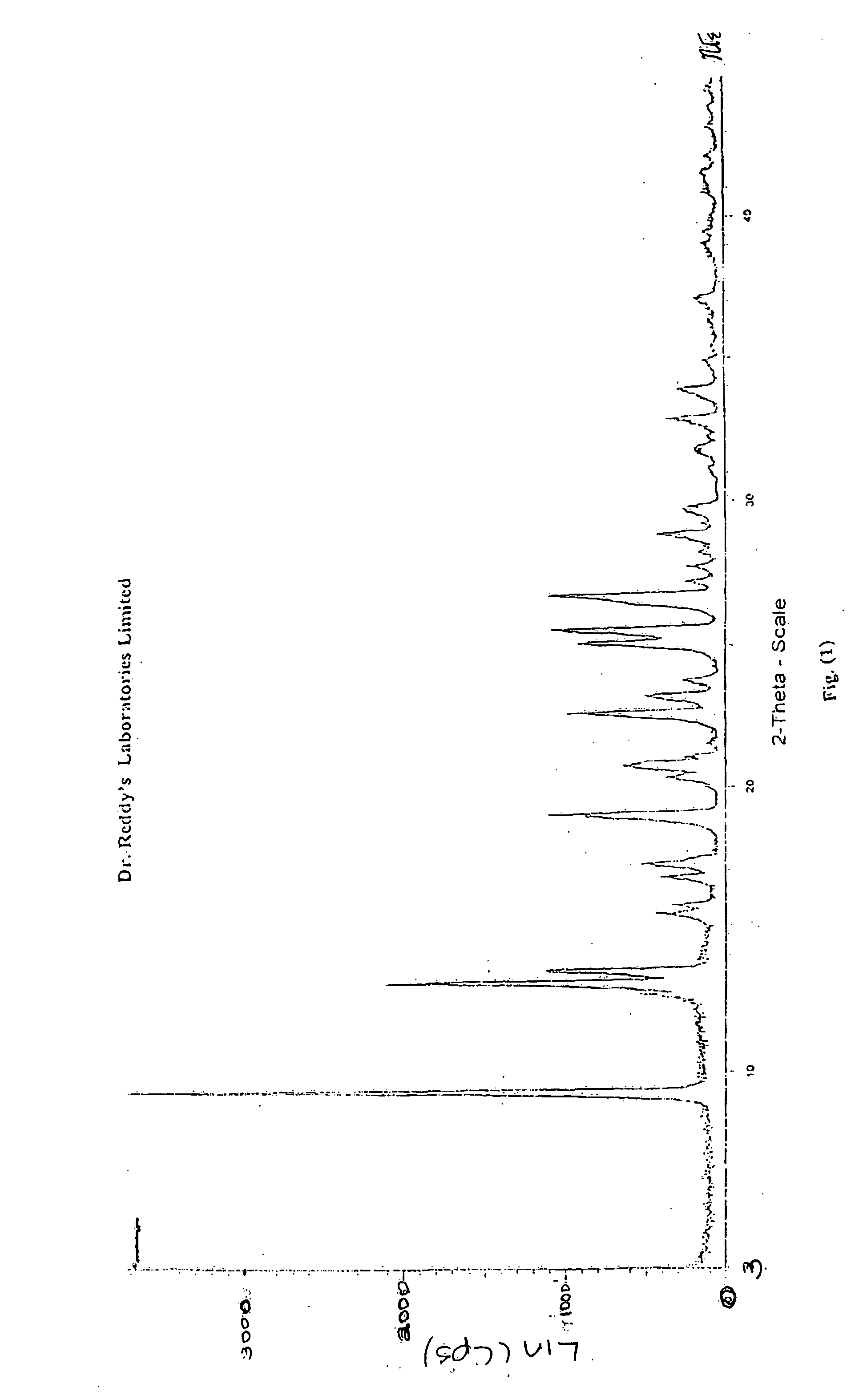
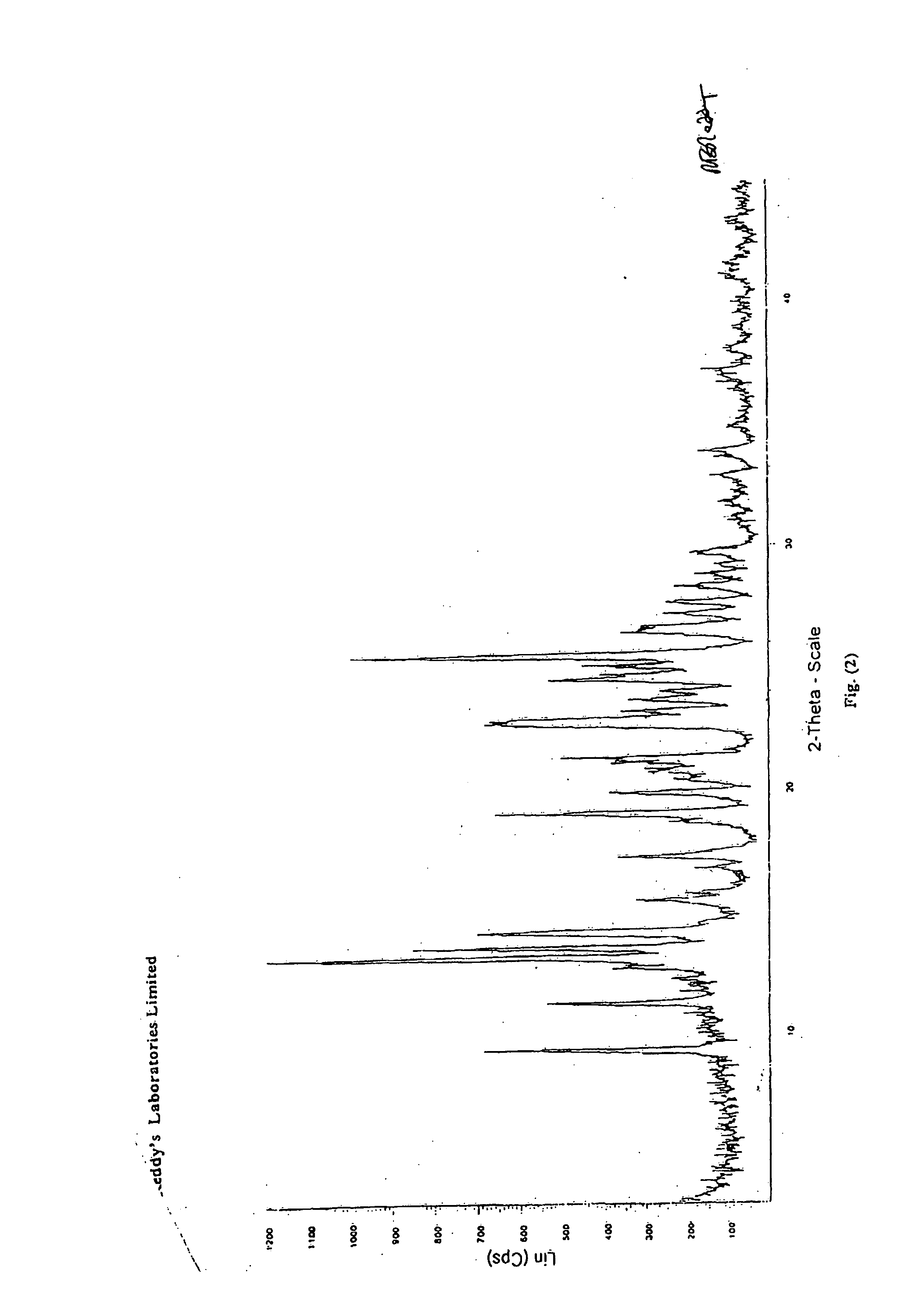
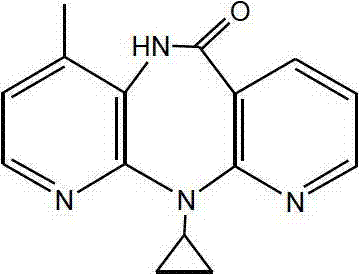
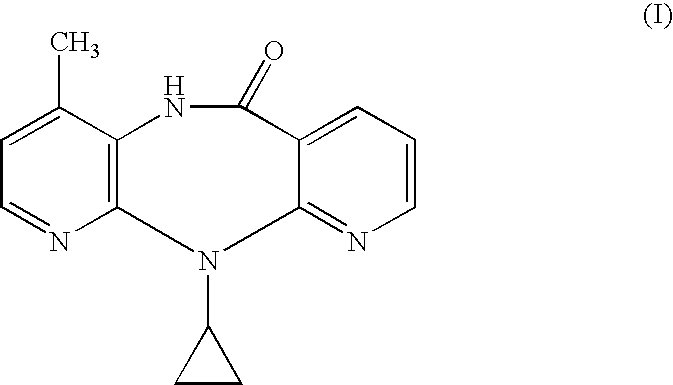
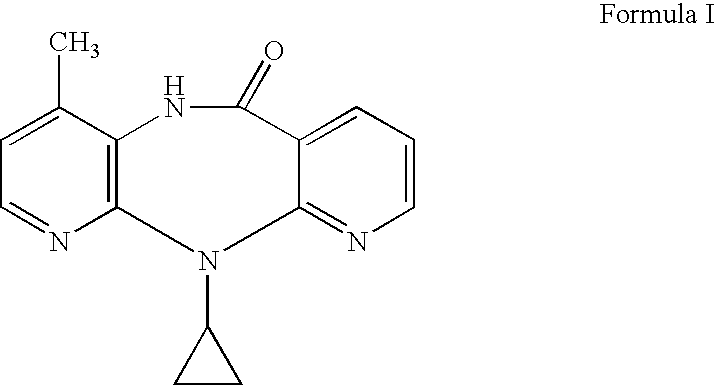
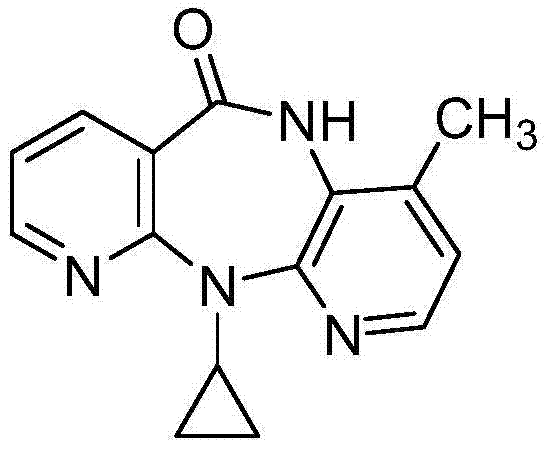
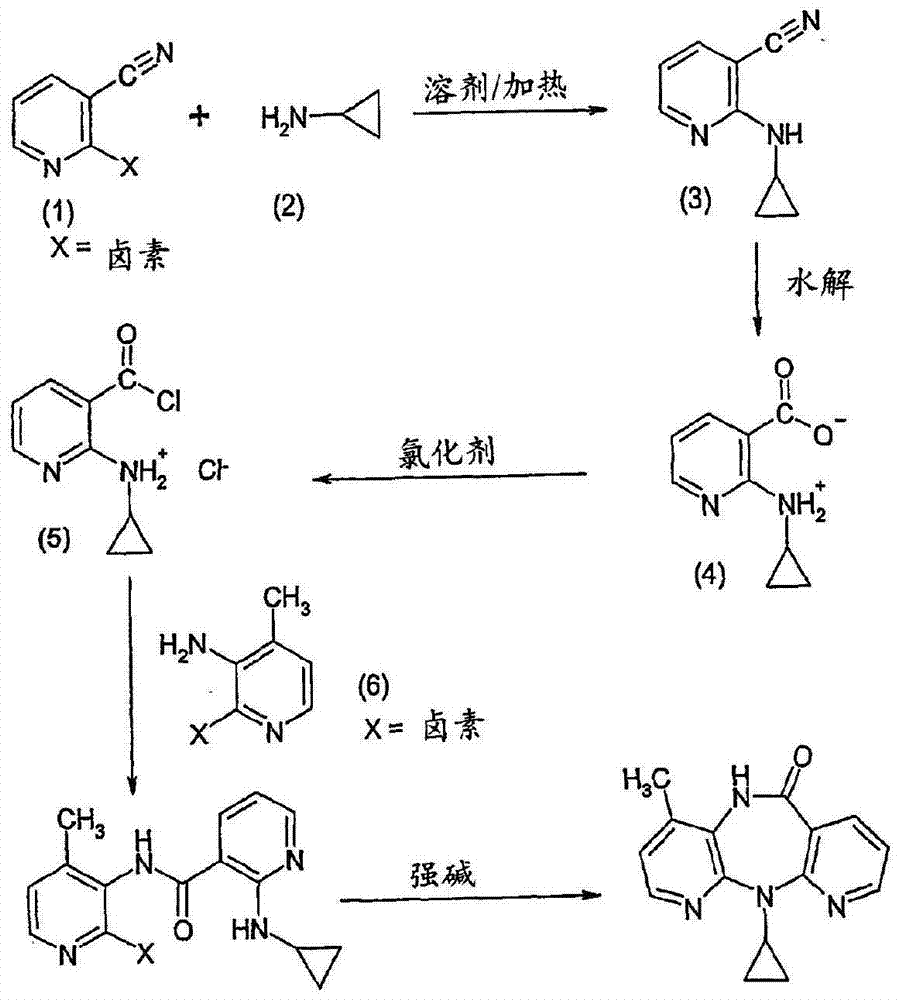
Novel crystalline forms of 11-cyclopropyl-5,11-dihydro-4-methyl-6H-dipyrido [3,2-b: 2',3'-e][1,4] diazepin-6-one (nevirapine)
InactiveUS20050059653A1Superior bioavailabiltyHigh activityBiocideOrganic chemistryMethyl groupCrystallization
The present invention relates to novel crystalline forms of 11-cyclopropyl1-5, 11-dihydro-4-methyl-6H-dipyrido[3,2-b: 2′, 3′-e][1,4]diazepin-6-one, generically known as Nevirapine, and processes for making thereof. More specifically, the present invention provides novel crystalline Form-II and Form-III of Nevirapine.
Owner:DR REDDYS LAB LTD
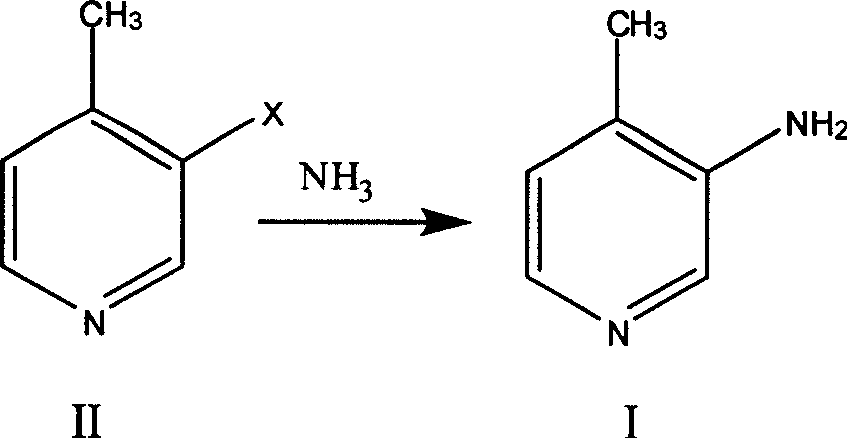
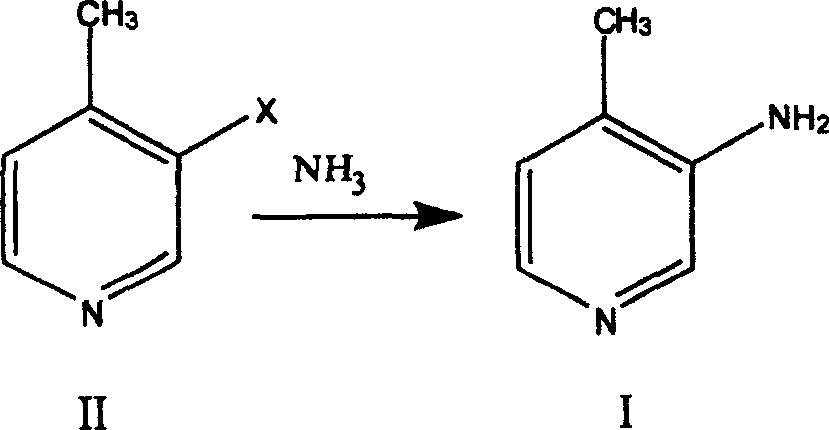
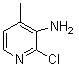
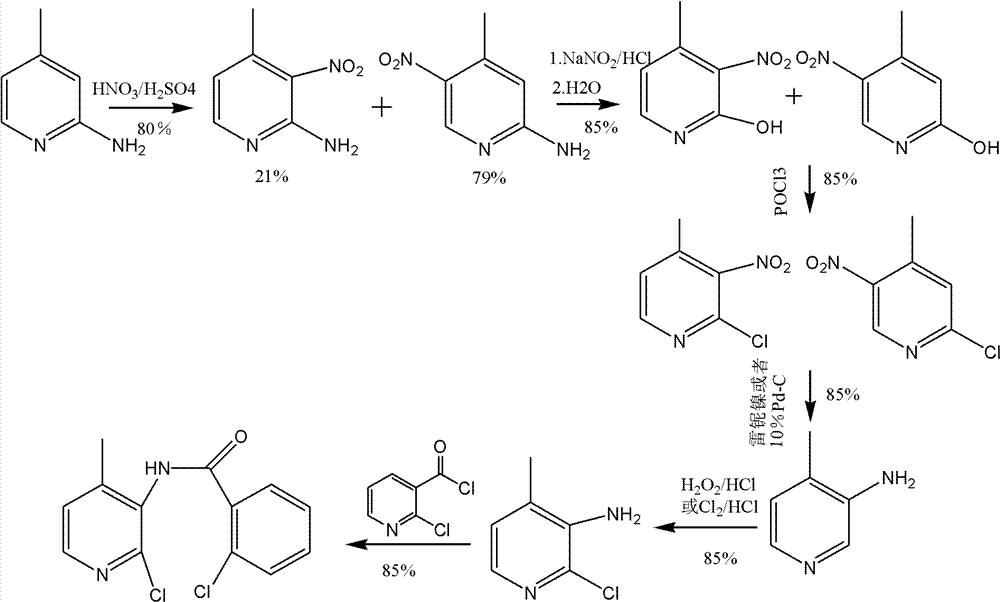
Preparation process of 3-amino-4 methyl pyridine
InactiveCN100460394CReduce manufacturing costHigh yieldOrganic chemistry4-MethylpyridineAnti aids drug
This invention relates to the field of pharmaceutical chemistry, involves the preparation method of Intermediate 3-amino-4-methylpyridine (1) of a specific anti-AIDS drugs nevirapine. The feature is it prepared by the 3-halogenated-4-methylpyridine, the preparation method is simple, mild conditions, and the yield is high.
Owner:CHINA PHARM UNIV +1
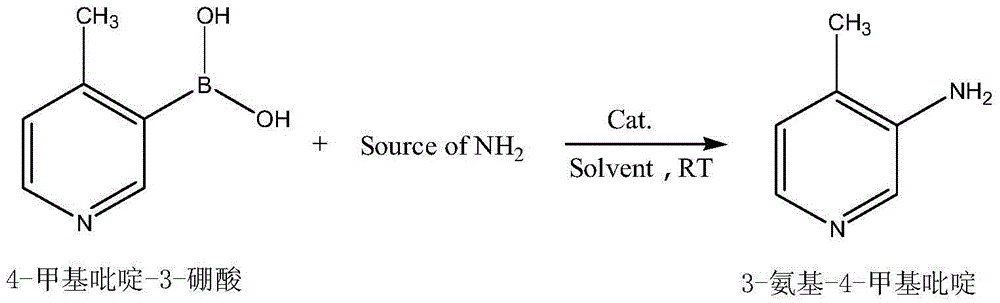
Preparation method of 3-amino-4-methylpyridine
ActiveCN104356057AThe reaction process is simpleHigh yieldOrganic chemistryChemical synthesisSynthesis methods
The invention belongs to the field of organic chemical synthesis and particularly relates to a synthesis method of 3-amino-4-methylpyridine which is an intermediate of an anti-AIDS drug nevirapine. According to the method, 4-methylpyridine-3-boronic acid is used as a raw material, an inorganic amide is used as an ammonia source and obtain3-amino-4-methylpyridine is prepared through one-step reaction in the presence of metal oxide as a catalyst. The preparation method is still simple and provides a new way for novel and efficient synthesis of 3-amino-4-methylpyridine and the disadvantages of long traditional route, low yield and severe reaction conditions are overcome.
Owner:JIANGSU ZHONGBANG PHARMA
Biomarker for forecasting severe drug-induced cutaneous adverse reaction of child patient and application
The invention discloses a biomarker for forecasting severe drug-induced cutaneous adverse reaction of a child patient and an application. The biomarker is capable of forecasting the risk of severe cutaneous adverse reaction of the child patient using beta-lactam antibiotics such as penicillin, cephalosporin, carbamazepine, lamotrigine, oxcarbazepine, phenytoin, allopurinol, nevirapine, abacavir, methazolamide and dapsone.
Owner:THE THIRD XIANGYA HOSPITAL OF CENT SOUTH UNIV
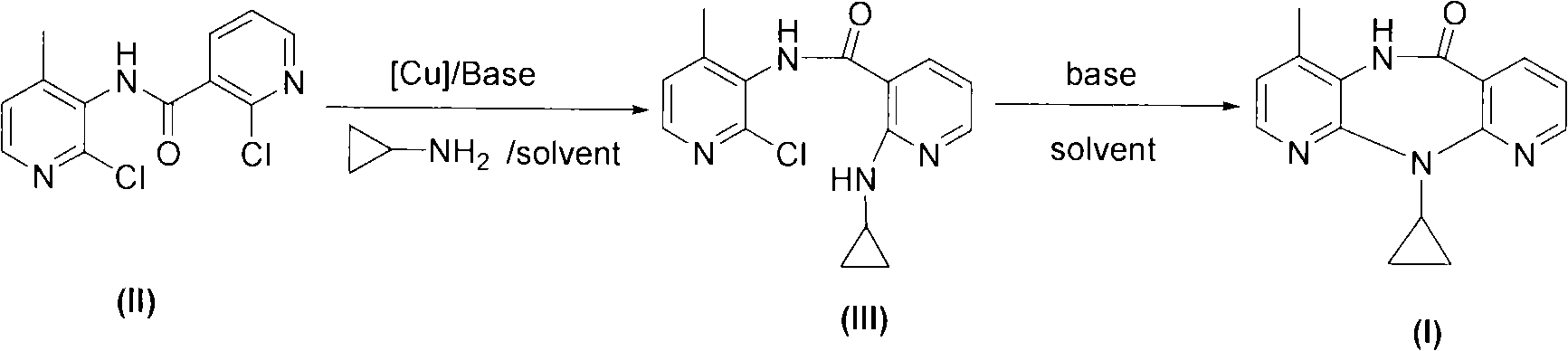
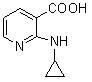
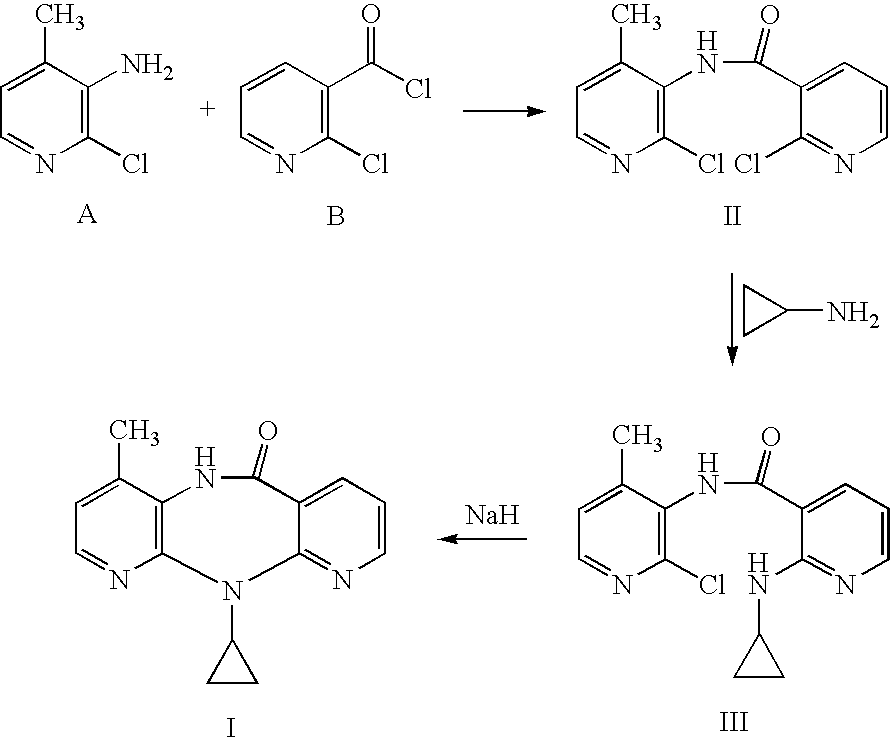
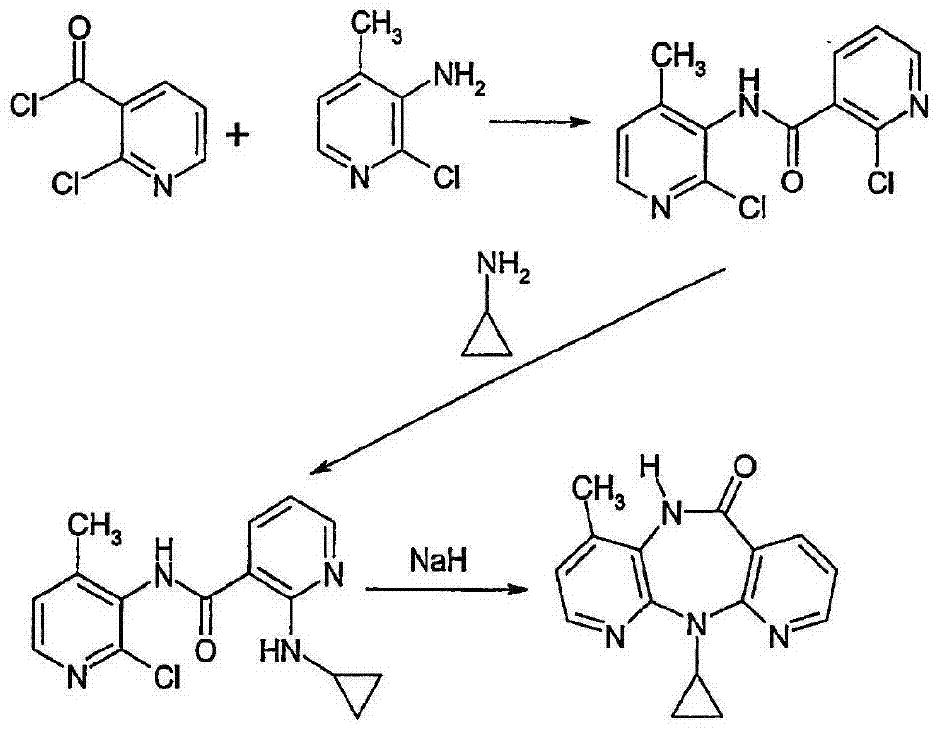
Novel method for preparing Nevirapine
The invention relates to a novel method for preparing Nevirapine and discloses a novel method for preparing 11-cyclopropyl-5,11-dihydrogen-4-methyl-6H-II naphthyridine (3,2-b:2',3'-e)(1,4) azatropylidene (I) starting from 2-chlorin-N-(2-chlorin-4-methyl-3-pyridyl)-3-pyridine carboxamide (II). The method comprises the steps: a compound (II) reacts with cyclopropylamine under the existence of organic solvent and alkali and the catalysis of cupric salt under normal temperature and pressure to produce a compound (III), and the obtained compound (III) produces 11-cyclopropyl-5,11-dihydrogen-4-methyl-6H-II naphthyridine (3,2-b:2',3'-e)(1,4) azatropylidene (I) after intramolecular cyclization under the action of a certain organic solvent and the alkali. The method has low cost, environmental protection and wild reaction condition, thereby being suitable for commercial process.
Owner:ZHEJIANG HUAHAI PHARMACEUTICAL CO LTD +1
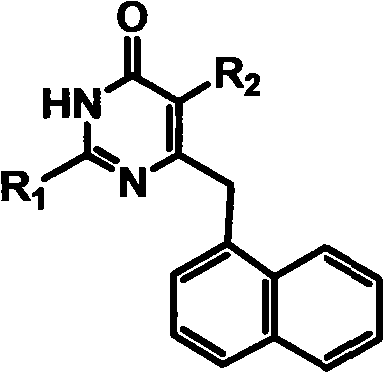
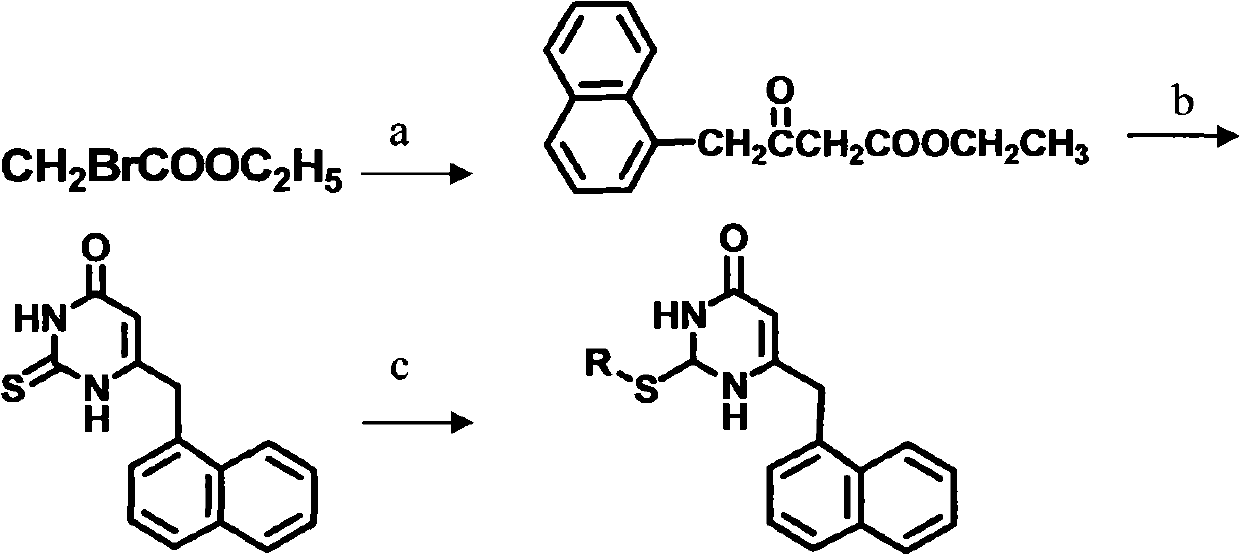
Preparation method and use of novel S-DABO HIV-1 reverse transcriptase inhibitor
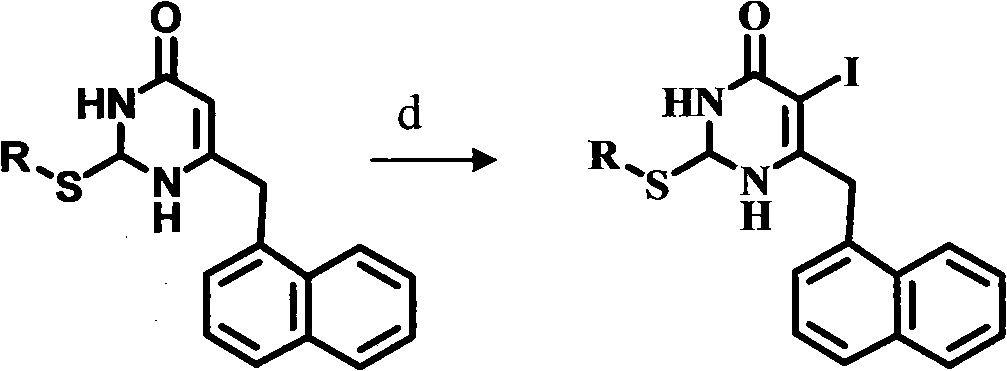
The invention relates to a novel S-DABO compound which is designed by taking non-nucleoside HIV-1 reverse transcriptase inhibitor 3,4- dihydro-2-alkoxy-6-benzyl-4- carbonyl pyrimidine (DABO) and 1-[(2- hydroxy ethoxy) methyl]-6- thiophenyl thymine (HEPT) as lead compounds, combining research results of the research team, connecting and merging each structure and activity relationship in a molecule according to a basic principle of reasonable drug design. The novel S-DABO compound is shown in the general formula I, and definition of each group is disclosed as description in an affidavit of claim. In the invention, the C2, C4 and C5 sites of a pyrimidine ring are modified through various different groups to obtain the novel compound which is more beneficial to be combined with HIV-1 reverse transcriptase with the structure. Through HIV RT activity test, the novel S-DABO HIV-1 reverse transcriptase inhibitor has higher activity, wherein the activity of one compound is higher than 23 times of nevirapine currently used in clinic; and the compound can become a novel class of latent drug for resisting AIDS.
Owner:CAPITAL UNIVERSITY OF MEDICAL SCIENCES
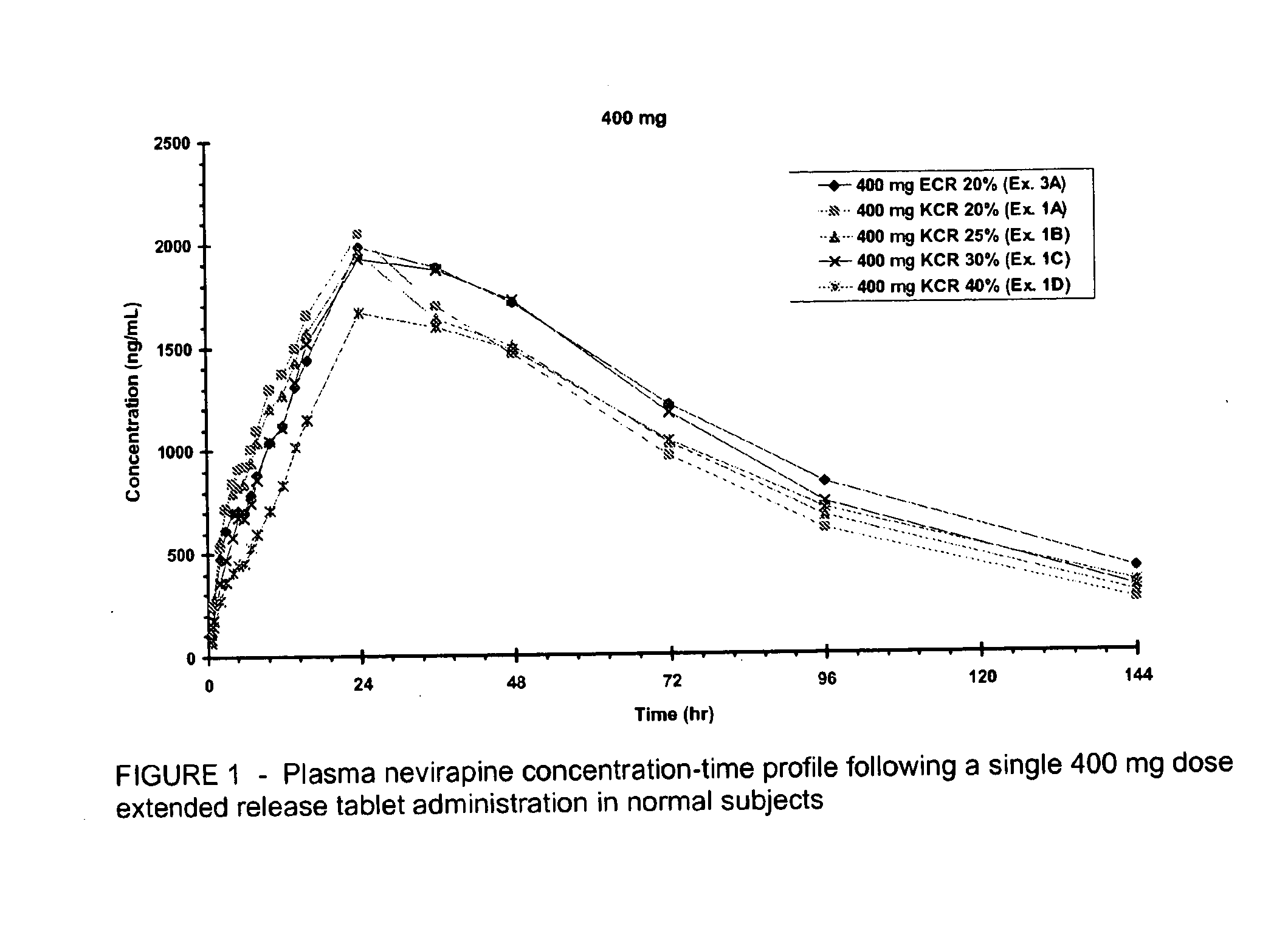
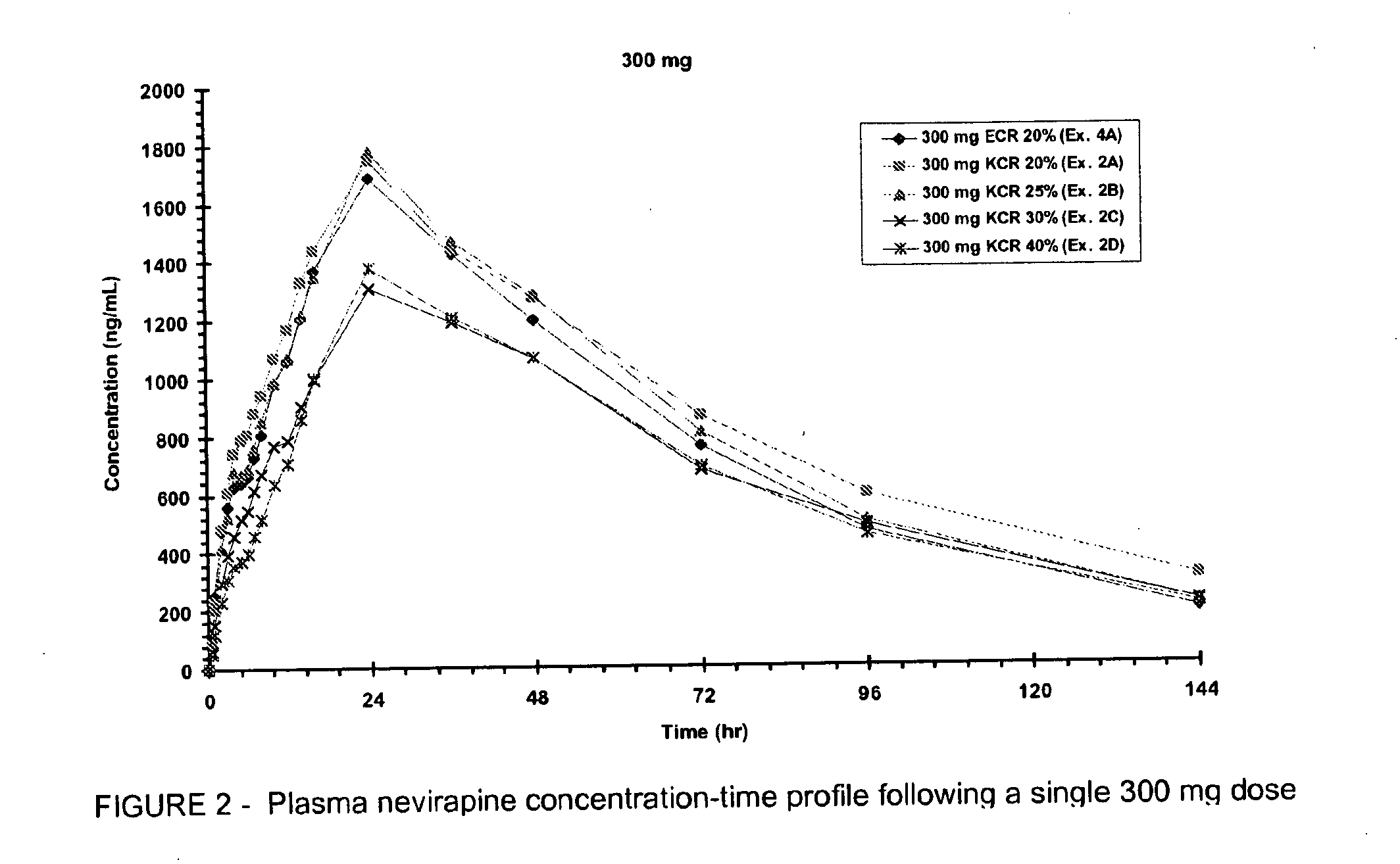
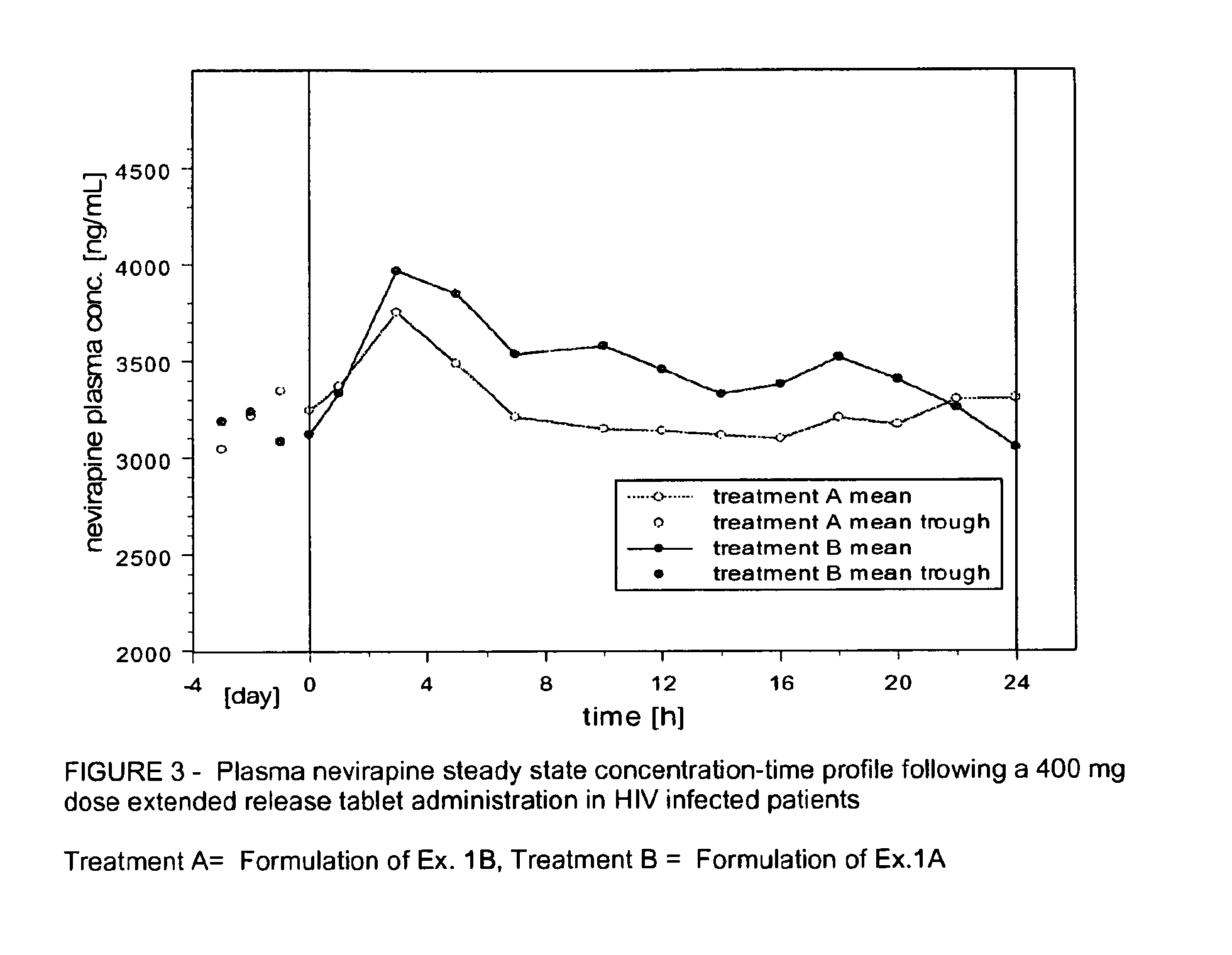
Extended release formulation of nevirapine
Owner:BOEHRINGER INGELHEIM INT GMBH
Method for preparing nevirapine
InactiveCN102127077AAvoid pollutionAvoid Adding Air Scrubbing EquipmentOrganic chemistryAntiviralsNiacinamideCarboxylic acid
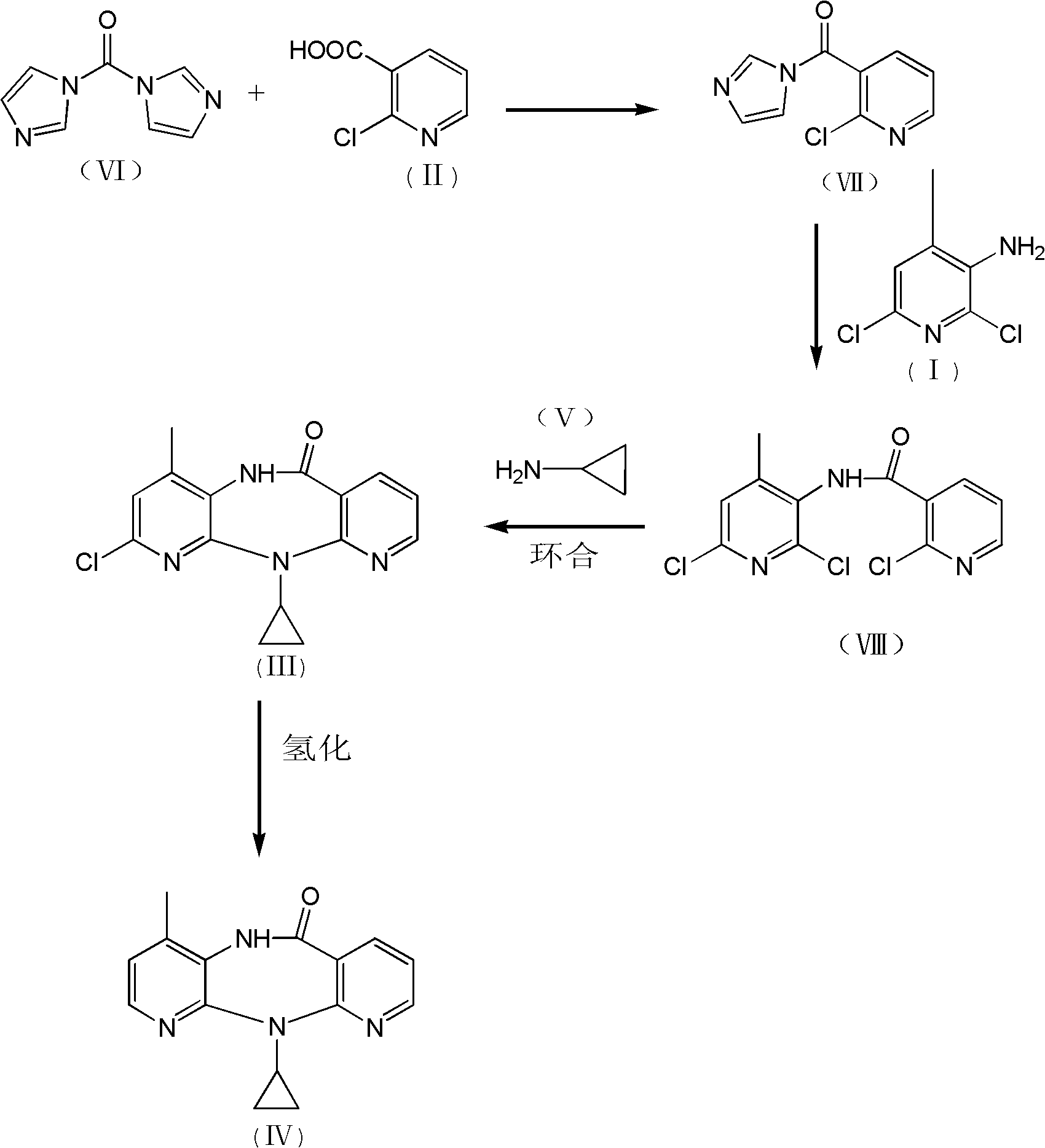
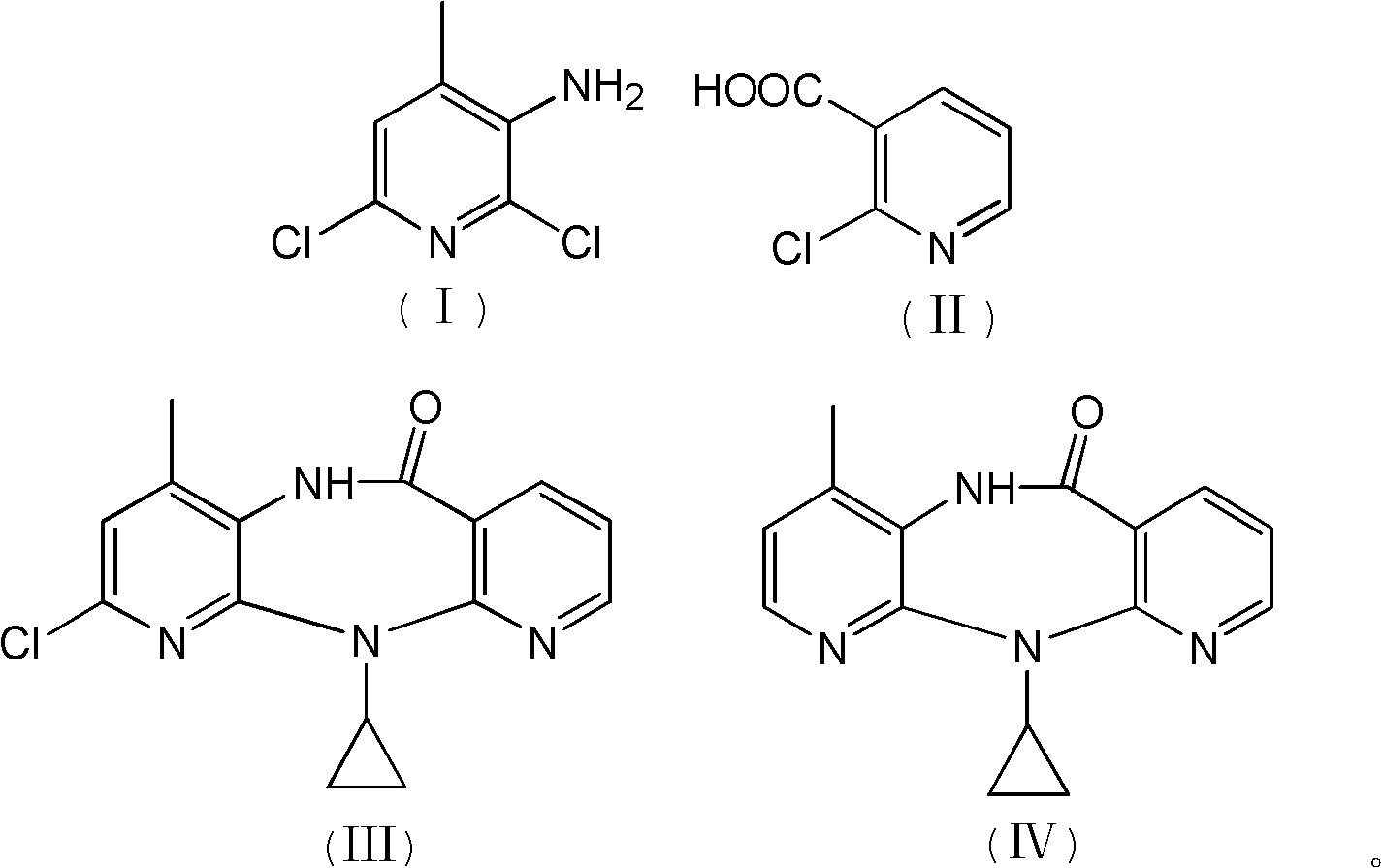
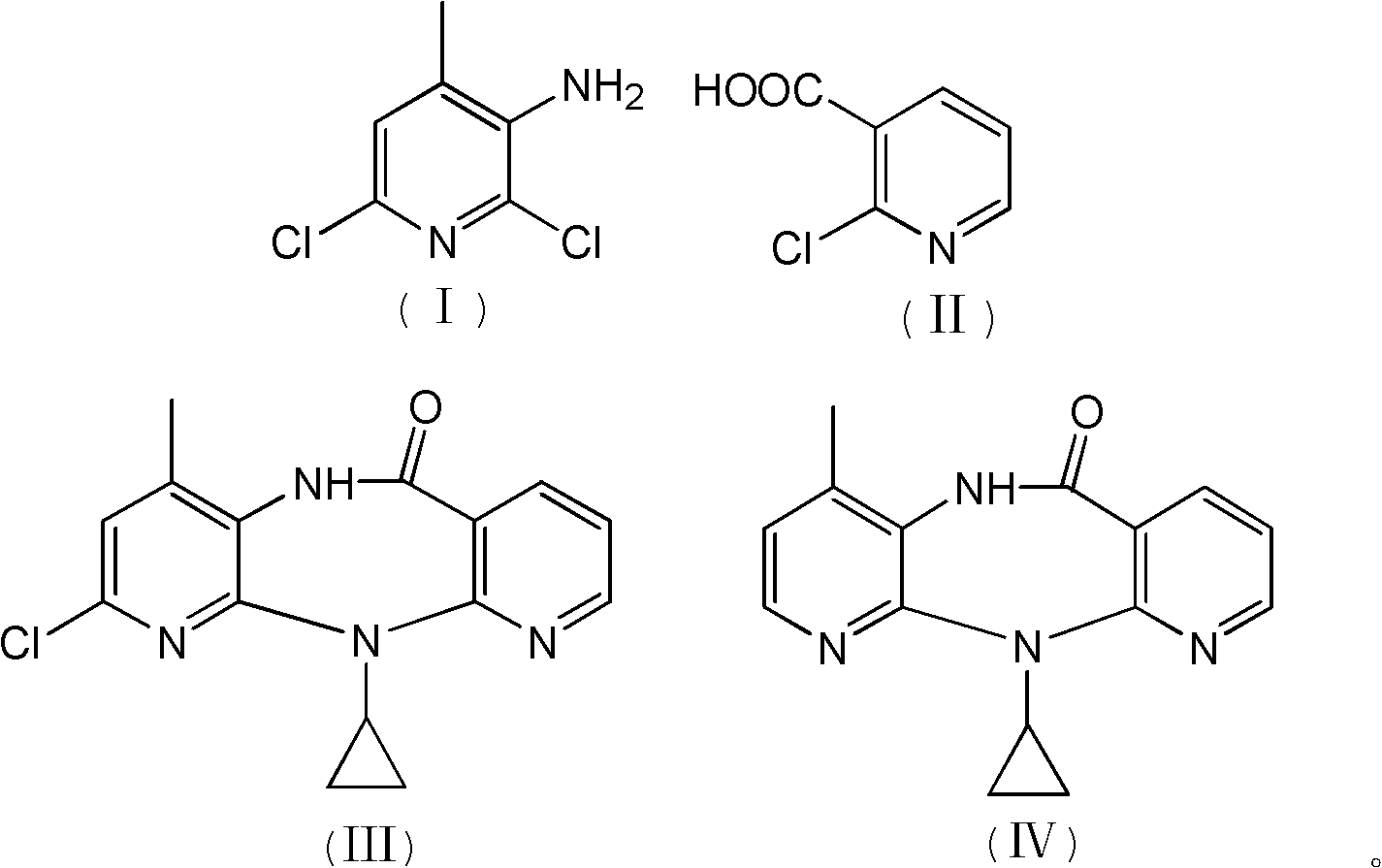
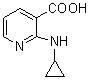
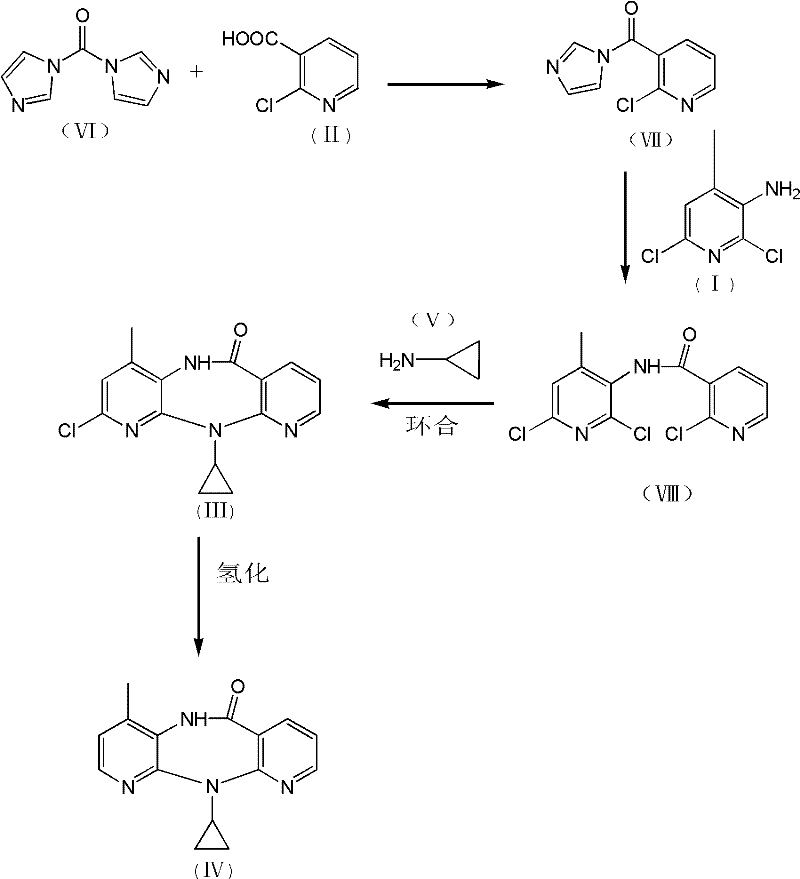
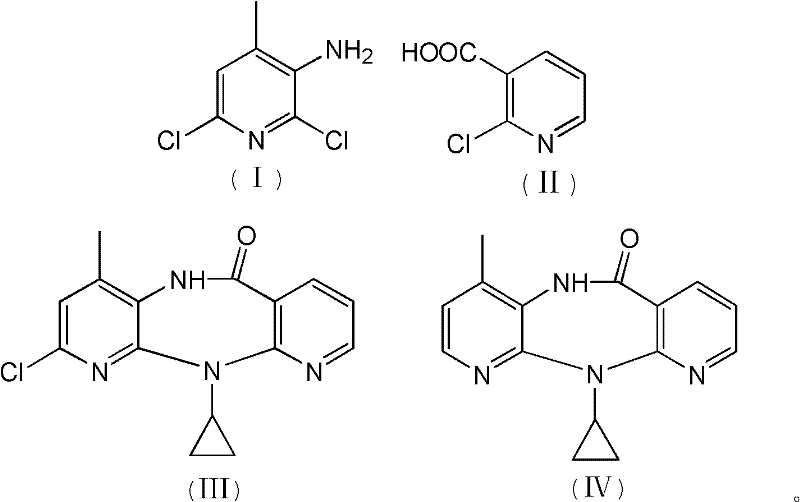
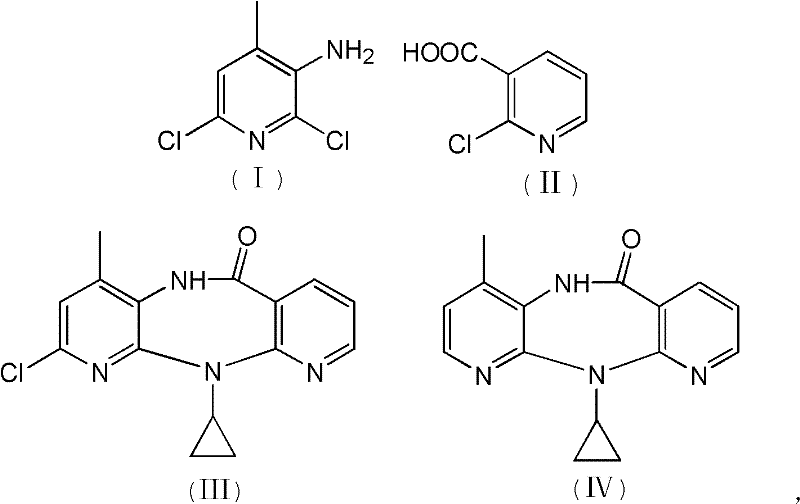
The invention discloses a method for preparing nevirapine, which comprises the steps of: A. enabling 2-chlorine-3 picolinic acid shown in the structural formula (II) and carbonyl diimidazole to react to produce active amide; B. enabling the active amide produced in the step A to react with 2, 6- dichloro-3-amino-4-methylpyridine shown in the structural formula (I), and producing N-(2, 6-dichloro-4-methyl-3-pyridyl)-2-chlorine niacinamide; C. carrying out nucleophilic substitution reaction on the N-(2, 6-dichloro-4-methyl-3-pyridyl)-2-chlorine niacinamide obtained in the step B and cyclopropylamine, then carrying out cyclization on the obtained products to obtain 2-chloro nevirapine with structure shown in the formula (III); and D. under the presence of catalyst, carrying out hydrogenation reaction on the 2-chloro nevirapine with structure shown in the formula (III) in the step C and non-hydrogen hydrogen source, to obtain the nevirapine with structure shown in the formula (IV). The invention aims at providing a method for preparing the nevirapine, which is low in cost, environment-friendly, higher in safety and suitable for production.
Owner:ZHONGSHAN BAILING BIOTECHNOLOGY CO LTD
Method for preparing nevirapine
InactiveCN102167699AAvoid hyperbaric reactionsReduce security risksOrganic chemistryNucleoside Reverse Transcriptase InhibitorDiimide
The invention discloses a method for preparing nevirapine, in particular a new method for preparing a non-nucleoside reverse transcriptase inhibitor of Nevirapine for treating human immunodeficiency virus (HIV). The method comprises the following steps of: reacting 2-chloronicotinic acid serving as an initial material with cyclopropanamine; performing amidation reaction with 2-chloro-3-amino-4-methyl pyridine in the presence of N-hydroxysuccinimide, dicyclohexylcarbodiimide and triethylamine; and closing a ring under the action of potassium tert-butoxide to obtain the Nevirapine. The method has the advantages of cheap and readily available raw materials, simple operation, light pollution and suitability for industrial production.
Owner:HUNAN BOAODE BIOPHARML TECH DEV
Preparation and application of novel chiral 3,4-dihydro-2-alkoxyl-6-benzyl-4-oxopyrimidine (S-DABO) human immunodeficiency virus (HIV)-1 reverse transcriptase inhibitor
InactiveCN102134223AOrganic active ingredientsOrganic chemistryNucleoside Reverse Transcriptase InhibitorMedicine
The invention relates to preparation and an application of a novel chiral 3,4-dihydro-2-alkoxyl-6-benzyl-oxopyrimidine (S-DABO) human immunodeficiency virus (HIV)-1 reverse transcriptase (RT) inhibitor. In the preparation, 3,4-dihydro-2-alkoxyl-6-benzyl-4-oxopyrimidine (DABO) is used as a lead compound combined with a research result of a discussion group, the structure and active relation of the lead compound are linked and merged in a molecule based on a medicament reasonable design basic principle, so that a novel S-DABO compound chiral 3,4-dihydro-2-alkoxyl-6-benzyl-4-oxopyrimidine as shown in a general formula I is designed, wherein in the formula I, definitions of groups are shown in claims. The preparation is focused on the fact that different groups at C2 and C5 positions of a pyrimidine ring are modified, so as to obtain novel compounds which are more favorable of combining with the HIV-1 RT. An HIV-1 RT activity test finds that the novel compounds have high activities, wherein the activity of a compound is 14 times higher than that of clinically used nevirapine. The compounds are likely to become potential novel anti-HIV medicaments.
Owner:CAPITAL UNIVERSITY OF MEDICAL SCIENCES
Pharmaceutical compositions of antiretrovirals
The present invention relates to the stable pharmaceutical dosage forms of combination of antiretroviral agents. More particularly, the present invention relates to stable pharmaceutical dosage forms of Lamivudine, Zidovudine and Nevirapine, prepared by granulation technology.
Owner:AUROBINDO PHARMA LTD
Pharmaceutical Antiretroviral Combinations Comprising Lamivudine, Festinavir and Nevirapine
InactiveUS20150104511A1Easy to manufactureBiocidePowder deliveryAcquired immunodeficiencyVirus-Retrovirus
The present invention relates to a pharmaceutical antiretroviral composition comprising lamivudine, festinavir and nevirapine, to a process for preparing such a composition and to the use of such a composition for the treatment and / or prophylaxis of diseases caused by retroviruses, especially acquired immune deficiency syndrome or an HIV infection.
Owner:CIPLA LTD
Anti-AIDS (Acquired Immune Deficiency Syndrome) compound preparation and preparation method thereof
The invention discloses an anti-AIDS (Acquired Immune Deficiency Syndrome) compound preparation and a preparation method thereof. In particular, the compound preparation comprises 1) safe zidovudine in effective amount for treatment or pharmaceutically acceptable derivatives; 2) safe lamivudine in effective amount for treatment or pharmaceutically acceptable derivatives; 3) safe nevirapine in effective amount for treatment or pharmaceutically acceptable derivatives; 4) a disintegrating agent; and 5) pharmaceutically acceptable carriers or auxiliary materials beside the disintegrating agent, wherein the content of the disintegrating agent is 4-15wt% of total weight the compound preparation. The compound preparation is quick to disintegrate and adequate to dissolve. The invention further discloses a method for preparing the compound preparation.
Owner:SHANGHAI DESANO BIO PHARM CO LTD +1
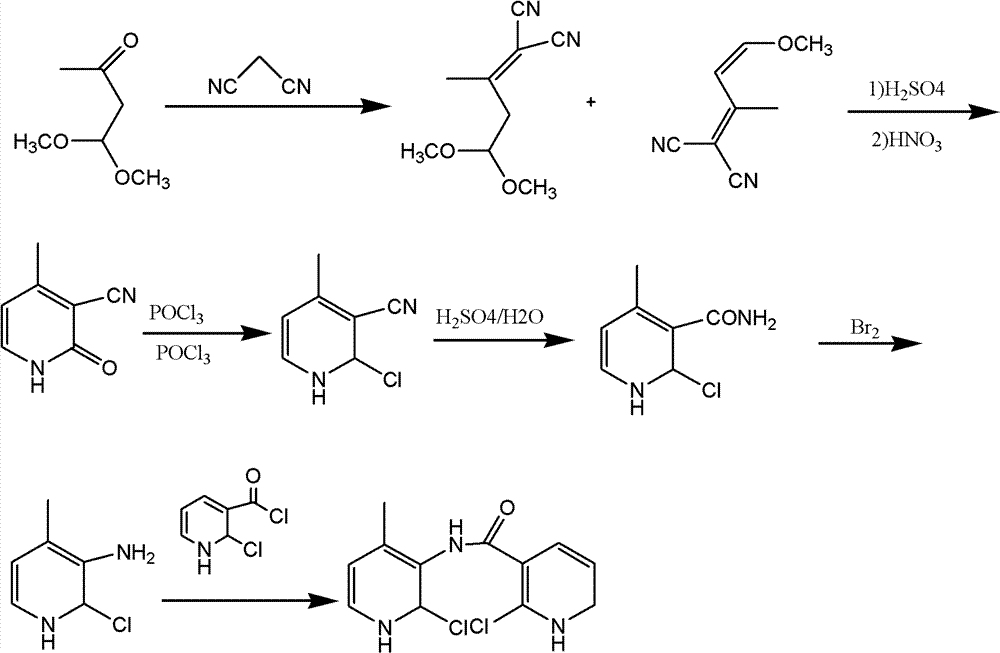
Method for preparing nevirapine
The invention relates to a method for preparing nevirapine. With the method disclosed by the invention, the nevirapine is obtained in four steps of Knoevenagel reaction, amination, copper salt catalytic reaction and cyclizing. The method has the advantages of simple technology and convenience in operation and is suitable for the large scale production of the modern enterprise, and the obtained nevirapine has an important influence on the development of the medicine field, and has a huge economic value and social value.
Owner:KAMP PHARMA
Viramune slice and its preparing method thereof
The invention discloses a nevirapine tablet and the related preparation method. The nevirapine tablet is made of 200 portions of nevirapine of 200, 120 to 150 portions of lactose, 50 to 70 portions of microcrystalline cellulose, 15 to 20 portions of sodium carboxy methyl starch, 4 to 5 portions of micronization silica gel, 3 to 4 portions of magnesium stearate and 2 to 3 portions of povidone K30 (based on weight). The invention has the advantages of high finished product rate and low cost.
Owner:ZHUHAI HUAAO IMPORT & EXPORT CORP
Preparation method of nevirapine intermediate spherical crystal
InactiveCN106938981ALow suspension densityImprove liquidityOrganic chemistry methodsTemperature controlState of art
The invention provides a preparation method of a nevirapine intermediate spherical crystal. The method concretely comprises the following steps: heating a reaction solution of a nevirapine intermediate 11-cyclopropyl-5,11-dihydro-4-methyl-6H-dipyrido-[3,2-b:2,3-e][1,4]diazepine to make the solution clarified, slowly dropwise adding the reaction solution to a crystallization solvent with the temperature controlled to be 10-40 DEG C, controlling the temperature of the crystallization system to be 0-50 DEG C during the dropwis adding, cooling the system to -10-10 DEG C after the dropwise adding is finished, filtering the solution, and drying the filtered solution to obtain the nevirapine intermediate spherical crystal. The method fills the gap in the prior art, the prepared spherical crystal has the advantages of small density and good fluidity of a crystal slurry, easiness in separation and drying, single solvent, low solvent rate, high crystallization yield, simple preparation method and high purity, and the preparation method has the advantages of easiness in commercial large-scale production, and very high promotion and application values.
Owner:ZHEJIANG HUAHAI PHARMACEUTICAL CO LTD
Crystalline forms of nevirapine
Owner:REGURI BUCHI REDDY +1
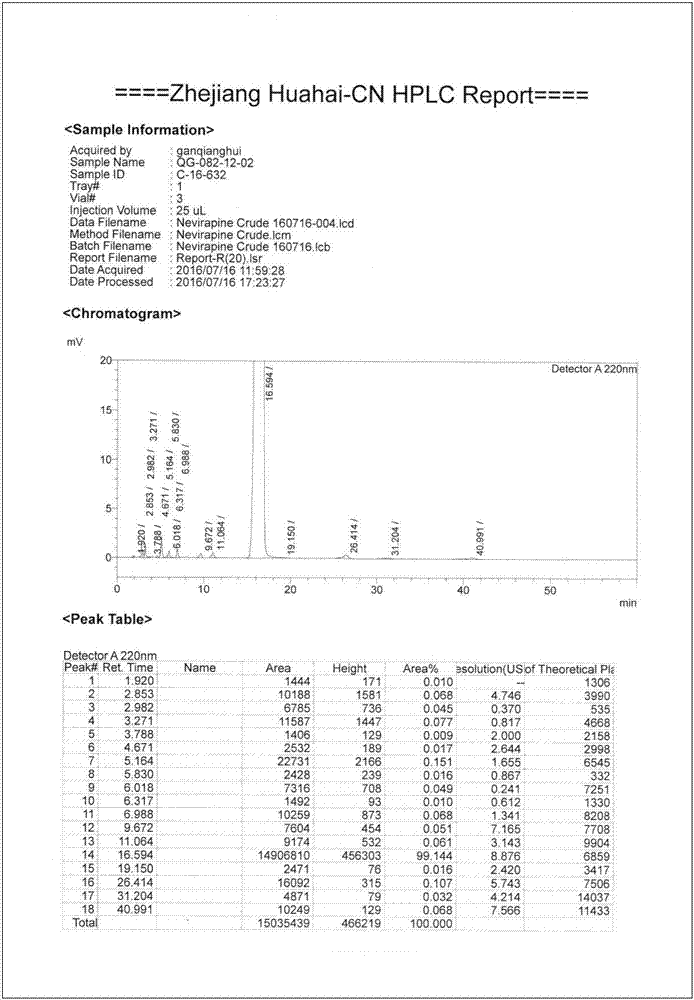
Method for separating and purifying nevirapine
ActiveCN102887898AThe process is simple and easy to controlQuality improvementOrganic chemistryOrganic solventCrystallization
The invention discloses a method for separating and purifying nevirapine. The method is characterized by comprising steps as follows: adding an organic solvent and a decolorizing agent to a crude product of nevirapine to dissolve and discolor, and distilling to remove the organic solvent which is 14-20 times the weight of the crude product of nevirapine, and then cooling to crystallize, so as to obtain a primary crystallized material of nevirapine; adding the organic solvent and the discoloring agent to the obtained primary crystallized material of nevirapine to dissolve and discolor, and distilling to remove the organic solvent which is 9-16 times the weight of the primary crystallized material of nevirapine, and then cooling to crystallize, so as to obtain a secondary crystallized material of nevirapine; adding the organic solvent and the discoloring agent to the secondary crystallized material of nevirapine to dissolve and discolor, and distilling to remove the organic solvent which is 4-10 times the weight of the secondary crystallized material of nevirapine, and then cooling to crystallize, so as to obtain a purified product of nevirapine. The method disclosed by the invention is simple, controllable, low in cost, high in quality of products, and convenient for achieve industrial production.
Owner:SICHUAN YIBIN WULIANGYE GROUP YIBIN PHARMA
Method for preparing nevirapine
InactiveCN102127077BSimple post-processingImprove hydrogenation yieldOrganic chemistryAntiviralsNiacinamideCarboxylic acid
The invention discloses a method for preparing nevirapine, which comprises the steps of: A. enabling 2-chlorine-3 picolinic acid shown in the structural formula (II) and carbonyl diimidazole to react to produce active amide; B. enabling the active amide produced in the step A to react with 2, 6- dichloro-3-amino-4-methylpyridine shown in the structural formula (I), and producing N-(2, 6-dichloro-4-methyl-3-pyridyl)-2-chlorine niacinamide; C. carrying out nucleophilic substitution reaction on the N-(2, 6-dichloro-4-methyl-3-pyridyl)-2-chlorine niacinamide obtained in the step B and cyclopropylamine, then carrying out cyclization on the obtained products to obtain 2-chloro nevirapine with structure shown in the formula (III); and D. under the presence of catalyst, carrying out hydrogenationreaction on the 2-chloro nevirapine with structure shown in the formula (III) in the step C and non-hydrogen hydrogen source, to obtain the nevirapine with structure shown in the formula (IV). The invention aims at providing a method for preparing the nevirapine, which is low in cost, environment-friendly, higher in safety and suitable for production.
Owner:ZHONGSHAN BAILING BIOTECHNOLOGY CO LTD
Process for preparing Nevirapine
Owner:AUROBINDO PHARMA LTD
Pharmaceutical Combinations Containing Lamivudine, Stavudine and Nevirapine
InactiveUS20080241265A1Improve efficiencyPrevent development of resistancePowder deliveryBiocideBody weightStavudine
A pharmaceutical composition comprising 10-120 mg lamivudine, 1-30 mg stavudine and 50-170 mg nevirapine for pediatric treatment of viral infections. One particularly preferred composition comprises a tablet containing 12 mg stavudine, 60 mg lamivudine and 100 mg nevirapine. Another particularly preferred composition comprises a second tablet containing 6 mg stavudine, 30 mg lamivudine and 500 mg nevirapine. These compositions are suitable for treating children having a body weight from 5 to 30 kg.
Owner:CIPLA LTD
Quadruple therapy useful for treating persons afflicted with the human immunodeficiency virus (HIV)
InactiveUS20150305983A1Reduce in quantityMaintain curative effectComputer controlDrug and medicationsEmtricitabineNucleotide
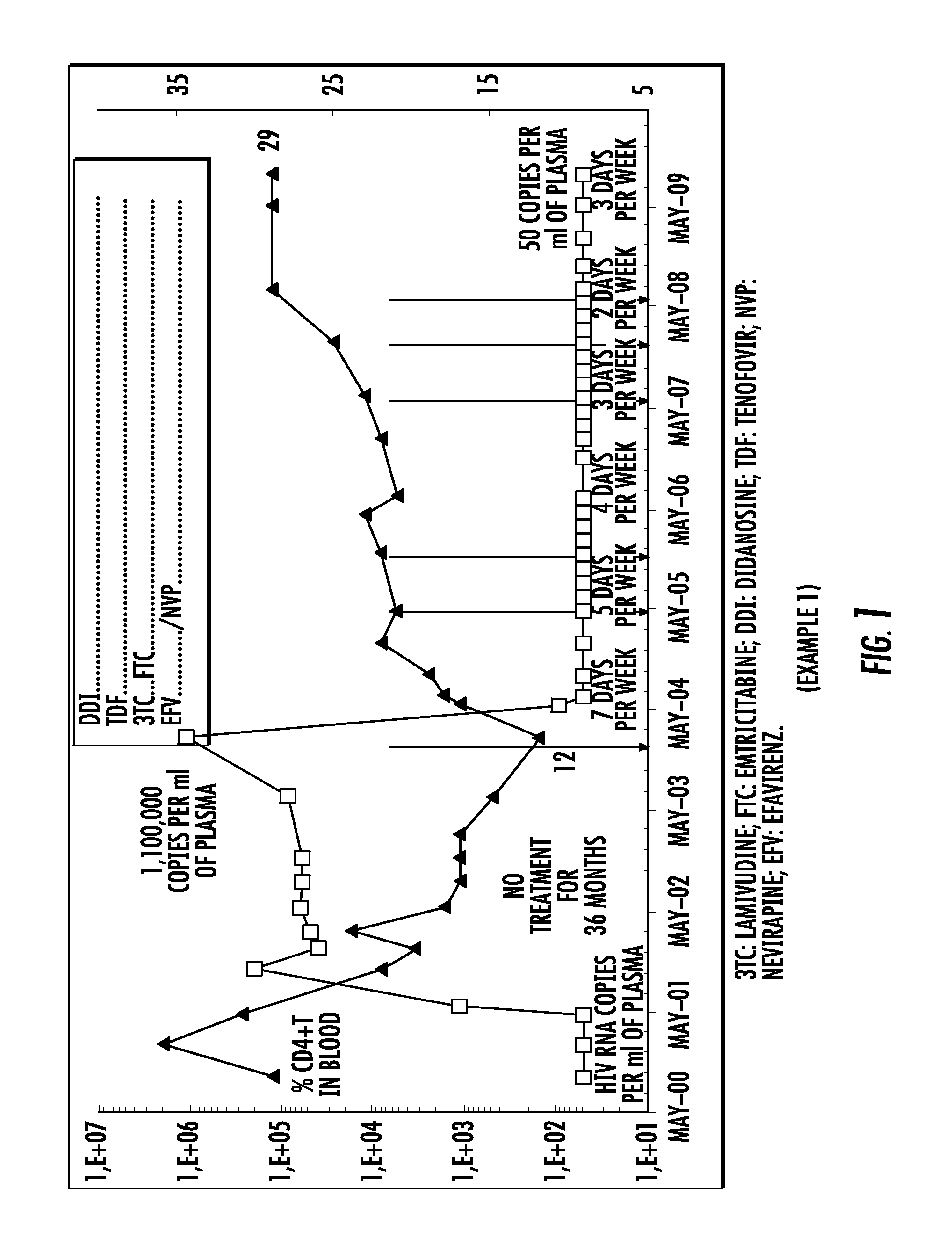
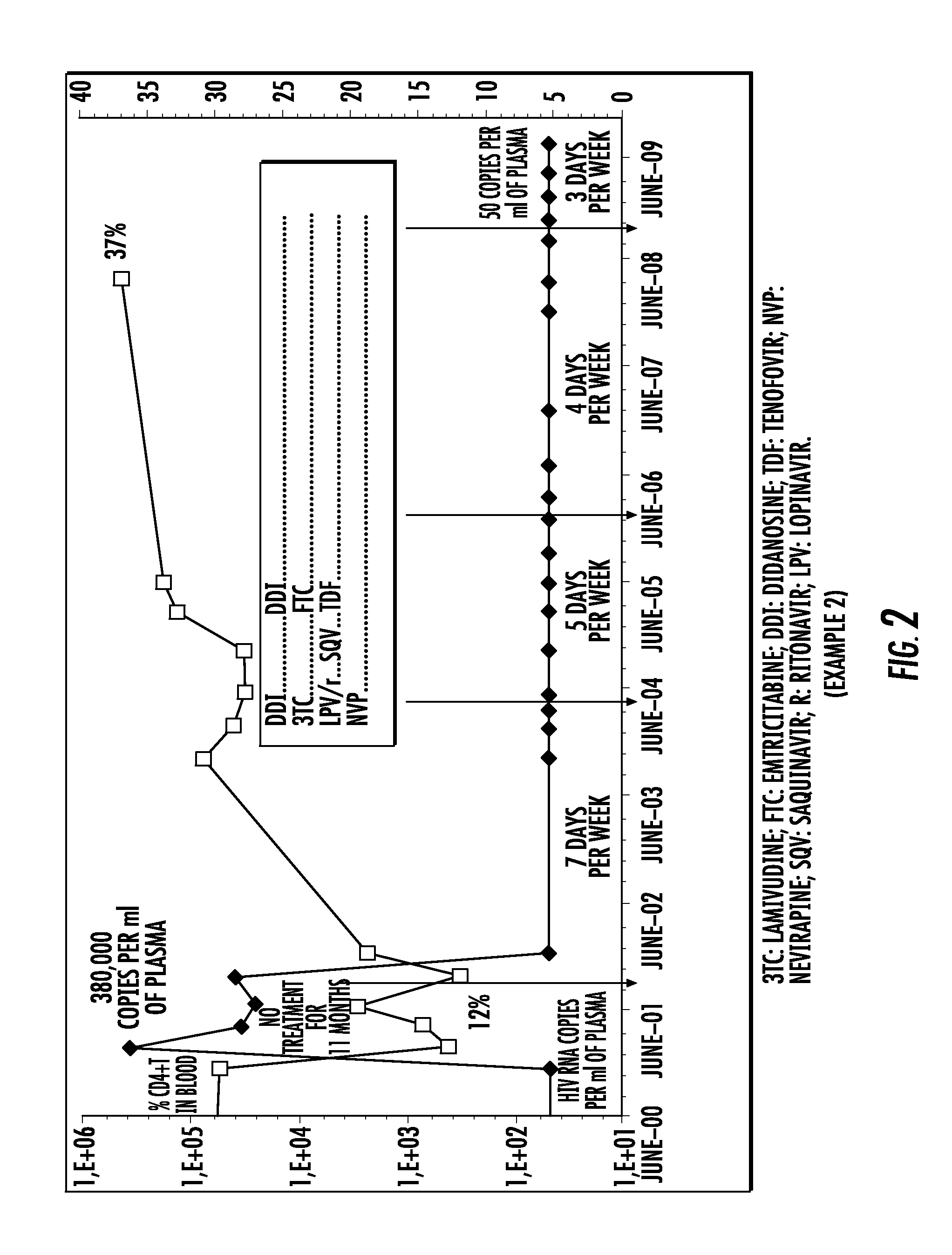
The present invention relates to a pharmaceutical composition for treating the human immunodeficiency virus (HIV) in a human being, comprising four active principles selected as being: one nucleoside inhibitor of reverse transcriptase (NRTI) selected from lamivudine and emtricitabine; two nucleoside or nucleotide inhibitor of reverse transcriptase (NRTI) selected from didanosine, abacavir and tenofovir; and the fourth active principle is selected from (i) a non-nucleoside inhibitor of reverse transcriptase (NNRTI) selected from nevirapine, efavirenz and etravirine; or (ii) a protease inhibitor selected from atazanavir, lopinavir, saquinavir, ritonavir, indinavir, amprenavir, nelfinavir, fosamprenavir, tipranavir and darunavir.The present invention also relates to an electronic portable pillbox comprising a multidrug therapy for treating the immunodeficiency virus (HIV) in human beings allowing improving the observance of medication intake.
Owner:UNIV VERSAILLES SAINT QUENTIN EN YVELINES
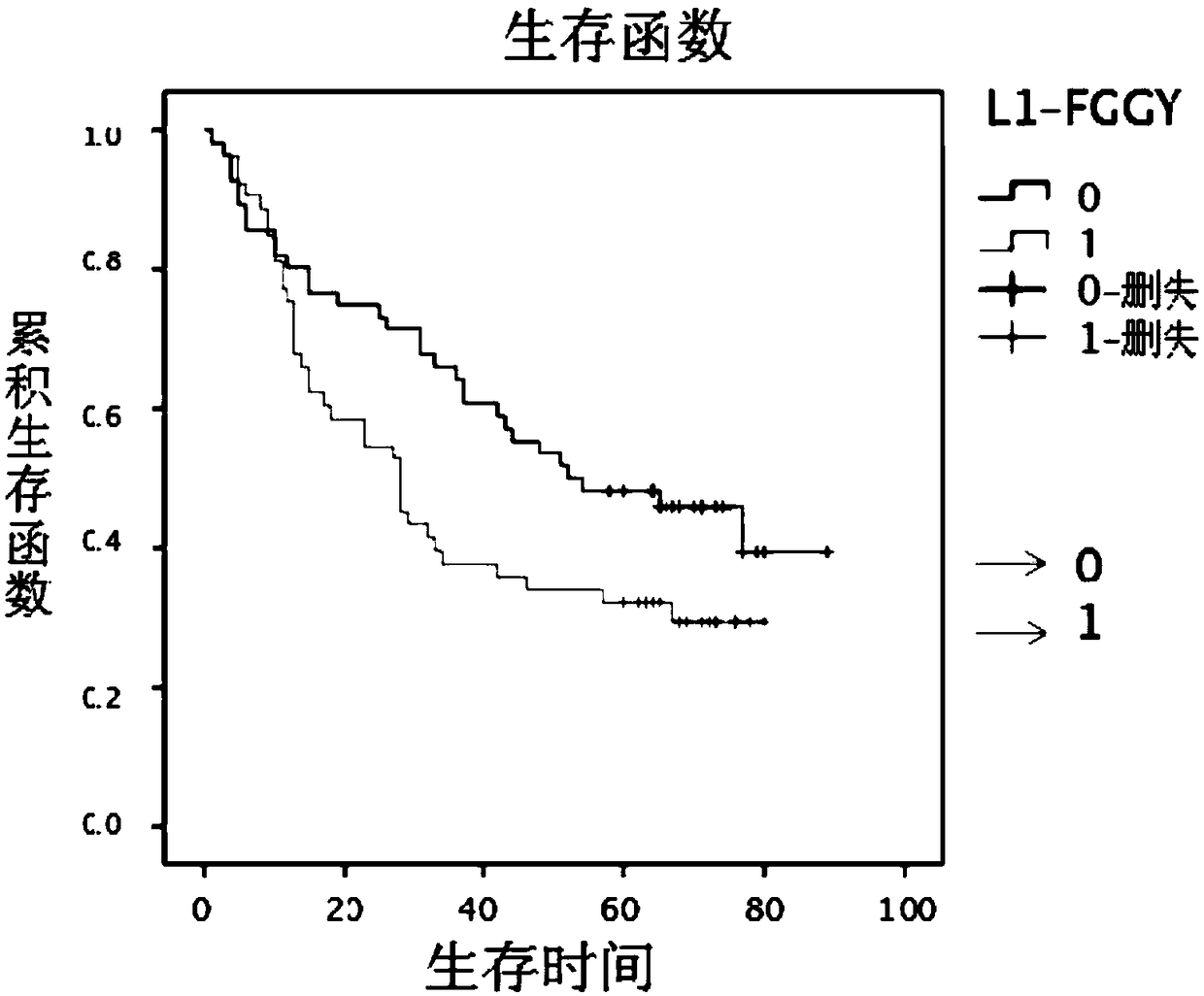
Retrotransposition gene L1-FGGY and application thereof as marker of lung squamous cell carcinoma
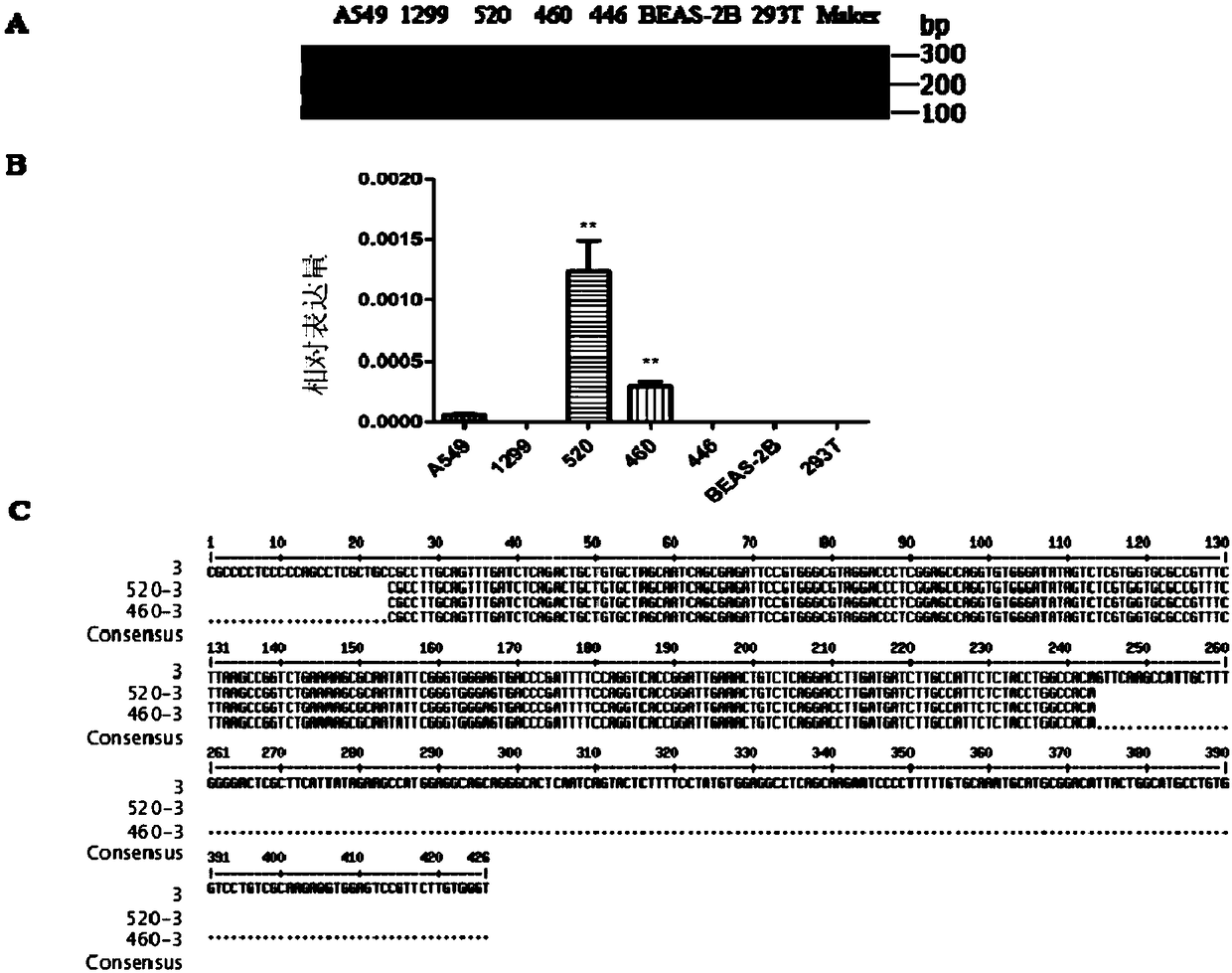
ActiveCN108796079AIncreased proliferationIncrease aggressivenessOrganic active ingredientsMicrobiological testing/measurementLung squamous cell carcinomaNucleic acid sequencing
The invention discloses retrotransposition gene L1-FGGY and application thereof as a marker of lung squamous cell carcinoma. The nucleic acid sequence of the retrotransposition gene L1-FGGY is SEQ IDNO. 1; the nucleic acid sequence of an upstream detection primer of the retrotransposition gene L1-FGGY is SEQ ID NO. 2; and the nucleic acid sequence of a downstream detection primer is SEQ ID NO. 3.A retrotransfer inhibitor nevirapine or efavirenz can inhibit the expression level of the retrotransposition gene L1-FGGY for treatment of lung squamous cell carcinoma. The retrotransposition gene L1-FGGY disclosed can be used as a new tumor marker, and the detection of L1-FGGY can be used for early diagnosis, molecular typing and prognosis evaluation of lung squamous cell carcinoma, and L1-FGGYcan also become a potential therapeutic target for clinical treatment of lung squamous cell carcinoma and has broad application prospects.
Owner:TIANJIN MEDICAL UNIV CANCER INST & HOSPITAL
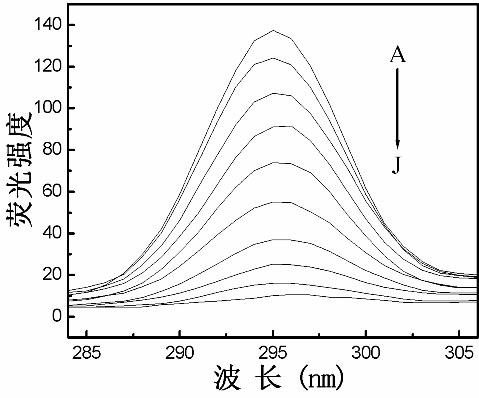
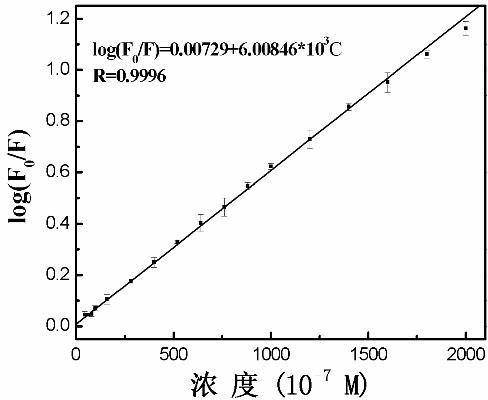
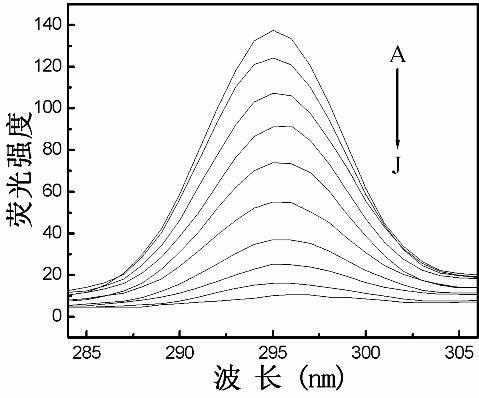
Method for determining Nevirapine by utilizing ZnS nano fluorescence probe
InactiveCN102435592AOptimizationRapid determinationFluorescence/phosphorescenceLuminescent compositionsFluoProbesChemical physics
The invention relates to a method for rapidly determining Nevirapine by utilizing mercaptoacetic-acid-modified ZnS nano particles as a nano fluorescence probe, and belongs to the technical field of optical analysis detection. In the method provided by the invention, a principle that the fluorescence intensity changes after the ZnS nano fluorescence probe interacts with Nevirapine to be determinedis mainly utilized, thus sensitive and quantitative analysis and determination are carried out on the Nevirapine through spectrofluorimetry. In the method provided by the invention, by optimizing experiment conditions such as temperature, addition sequence, pH value and reaction time, the fluorescence probe is applied to quantitative detection of the Nevirapine in optimal conditions. The determination process provided by the invention has the characteristics of simpleness, rapidness, sensitivity, accuracy and the like.
Owner:SHANGHAI UNIV
Methods and compositions for determining altered susceptibility of HIV-1 to anti-HIV drugs
ActiveUS20090136915A1Microbiological testing/measurementDead animal preservationNucleoside Reverse Transcriptase InhibitorDelavirdine
This invention relates, in part, to methods and compositions for determining altered susceptibility of a human immunodeficiency virus (“HIV”) to the non-nucleoside reverse transcriptase inhibitors (“NNRTIs”) efavirenz (“EFV”), nevirapine (“NVP”), and delavirdine (“DLV”), the nucleoside reverse transcriptase inhibitor AZT, and the integrase strand transfer inhibitors diketo acid 1, diketo acid 2, and L-870,810 by detecting the presence of a mutation or combinations of mutations in the gene encoding HIV reverse transcriptase that are associated with altered susceptibility to the anti-HIV drugs.
Owner:MONOGRAM BIOSCIENCES
Risk assessment for cutaneous adverse drug reactions from antiretroviral agent
ActiveUS20110301043A1Microbiological testing/measurementDisease diagnosisNucleoside Reverse Transcriptase InhibitorHLA-B
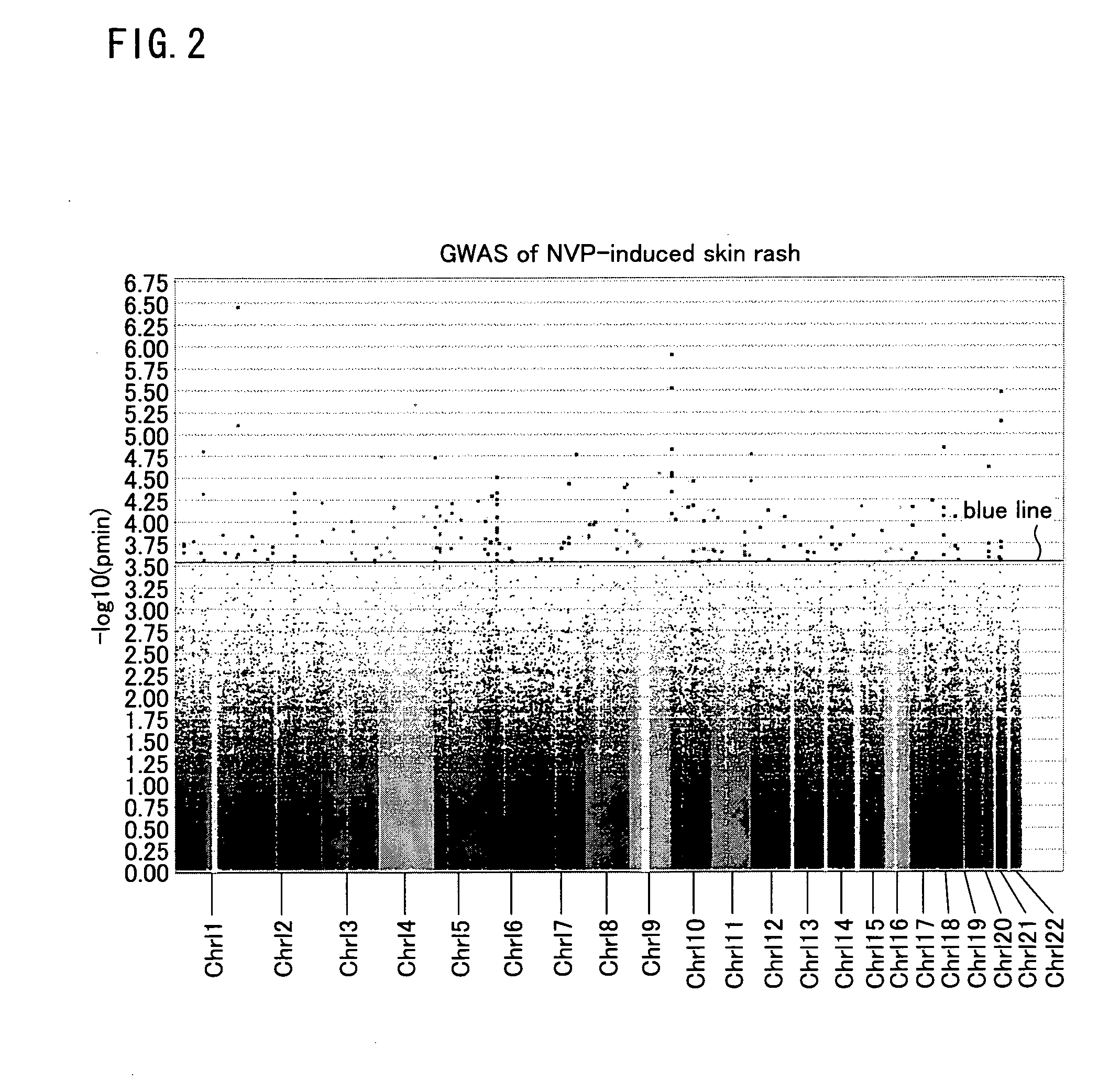
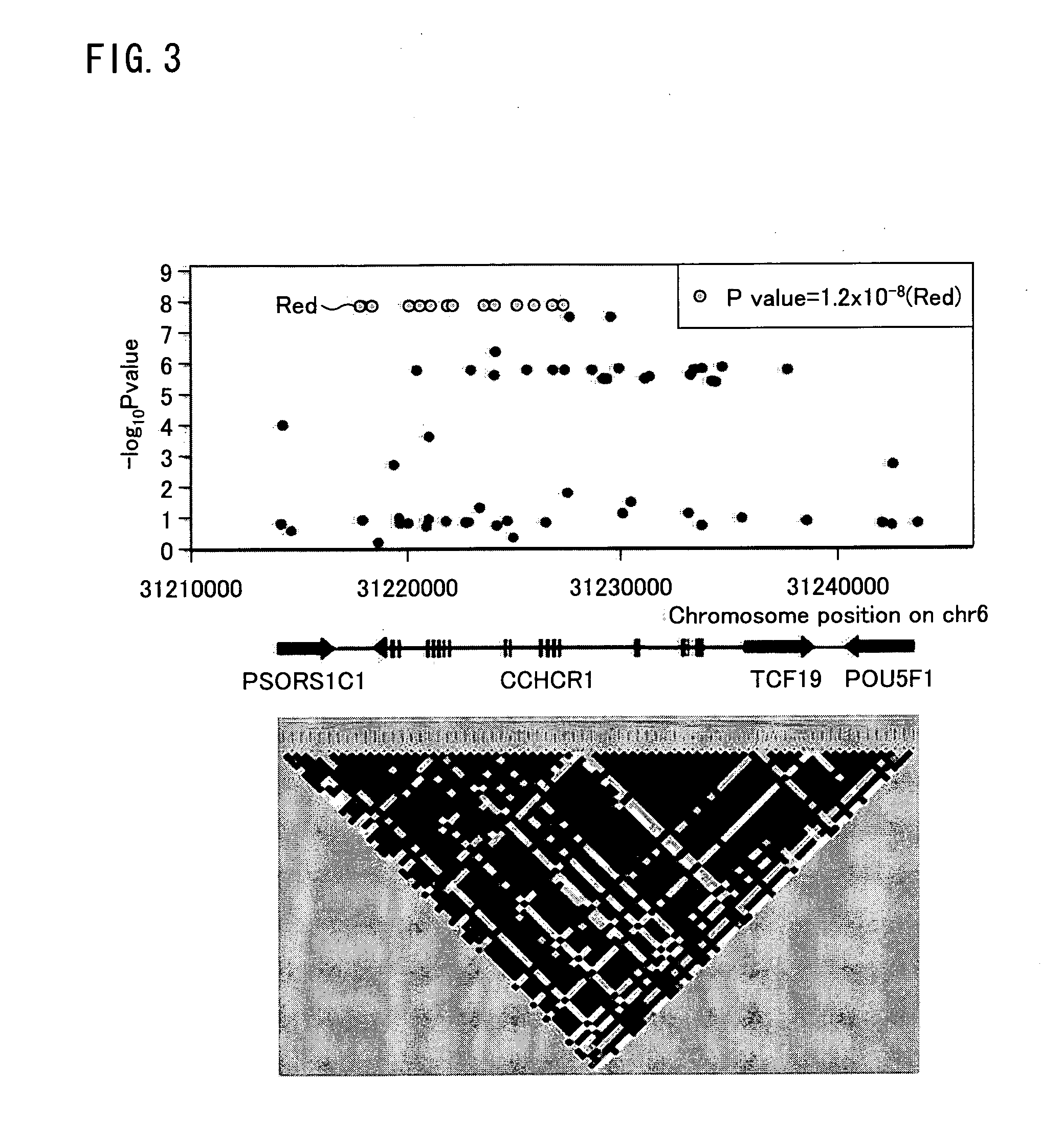
The present invention provides a method of predicting the risk of a patient for developing cutaneous adverse drug reaction to non-nucleoside reverse transcriptase inhibitors such as nevirapine by using HLA-B*3505 allele and / or polymorphisms in the CCHCR1 gene.
Owner:MAHIDOL UNIV +3
Methods for preparation of nevirapine and intermediate thereof
The invention provides methods for high-purity, high-yield, safe and effective preparation of nevirapine and an intermediate thereof. The method for preparation of the intermediate comprises the following specific steps: catalyzing a compound (II) 2-chloro-N-(2-chloro-4-methyl-3-pyridyl)-3-pyridyl amide by KI in the presence of an organic solvent and an alkali, carrying out a reflux reaction with cyclopropylamine, and thus obtaining a compound (III) N-(2-chloro-4-methyl-3-pyridyl)-2-(cyclopropyl amino)-3-pyridinecarboxamide. In the process of undergoing the reaction of the compound (II) with cyclopropylamine, the KI is added as the catalyst, the heating reflux reaction is carried out to obtain the compound (III), harsh high-temperature and high-pressure reaction conditions can be effectively avoided, risk factors brought by the need for high-pressure reaction kettles and high temperatures are overcome, and the preparation methods are safe and effective, and are convenient to operate.
Owner:LUNAN BETTER PHARMA
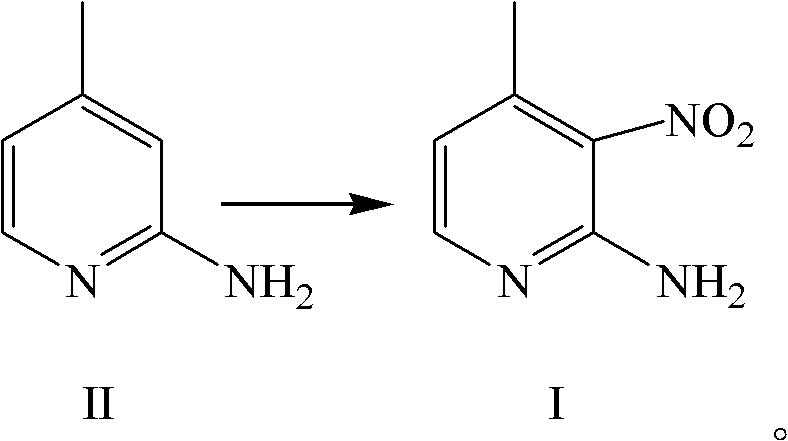
Synthesis method for Nevirapine intermediates
The invention discloses a synthesis method of a nevirapine intermediate 2-amino-3-nitro-4-methylpyridine shown as the chemical formula I. The synthesis method includes steps: under catalysis of inorganic base or organic base and actions of concentrated sulphuric acid and dilute nitric acid, subjecting the 2-amino-3-nitro-4-methylpyridine shown as the chemical formula I to nitration reaction in organic solvent. The inorganic base can be calcium hydroxide, barium hydroxide, sodium hydroxide or zinc hydroxide; and the organic base can be sodium methoxide, potassium methoxide, sodium ethoxide, potassium ethoxide, sodium tert-butoxide or potassium tert-butylate. The invention further discloses a synthesis method of a nevirapine intermediate 2-chloro-N-(2-chloro-4-methylpyridin-3-yl)nicotinamide. The synthesis method is easy and safe in operation, high in yield, low-cost, low in pollution to environments and suitable for industrial production in scale.
Owner:上海思协化工科技有限公司
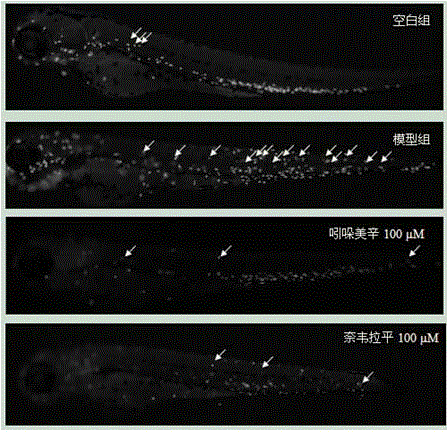
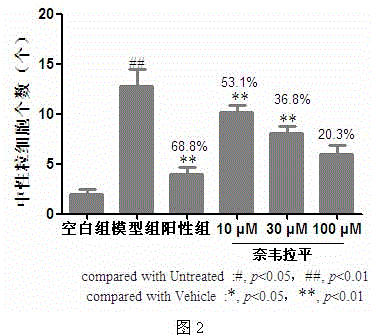
Application of nevirapine in preparing anti-inflammatory medicine
InactiveCN105456273AFacilitate resolutionEnhance anti-inflammatoryOrganic active ingredientsNervous disorderDiseasePharmaceutical drug
The invention belongs to the technical field of medicine and particularly relates to an application of nevirapine in preparing medicine for preventing and treating inflammation related diseases. Inflammation is aseptic inflammation and immune inflammation. The application of nevirapine in preparing the anti-inflammatory medicine is disclosed for the first time. Through experiment research on a copper sulfate induced zebra fish inflammation model, the result shows that nevirapine can promote degradation of neutrophile granulocyte of inflammatory sites and has anti-inflammatory and inflammation diminishing effects. Thus, nevirapine has the obvious anti-inflammatory effect on the inflammation related diseases and can be used for preparing the medicine for preventing and treating the inflammation related diseases.
Owner:HANGZHOU LEISUO PHARMA
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com
![Novel crystalline forms of 11-cyclopropyl-5,11-dihydro-4-methyl-6H-dipyrido [3,2-b: 2',3'-e][1,4] diazepin-6-one (nevirapine) Novel crystalline forms of 11-cyclopropyl-5,11-dihydro-4-methyl-6H-dipyrido [3,2-b: 2',3'-e][1,4] diazepin-6-one (nevirapine)](https://images-eureka.patsnap.com/patent_img/0360bf55-c604-4194-8700-18f50d0e9117/US20050059653A1-20050317-D00001.png)
![Novel crystalline forms of 11-cyclopropyl-5,11-dihydro-4-methyl-6H-dipyrido [3,2-b: 2',3'-e][1,4] diazepin-6-one (nevirapine) Novel crystalline forms of 11-cyclopropyl-5,11-dihydro-4-methyl-6H-dipyrido [3,2-b: 2',3'-e][1,4] diazepin-6-one (nevirapine)](https://images-eureka.patsnap.com/patent_img/0360bf55-c604-4194-8700-18f50d0e9117/US20050059653A1-20050317-D00002.png)
![Novel crystalline forms of 11-cyclopropyl-5,11-dihydro-4-methyl-6H-dipyrido [3,2-b: 2',3'-e][1,4] diazepin-6-one (nevirapine) Novel crystalline forms of 11-cyclopropyl-5,11-dihydro-4-methyl-6H-dipyrido [3,2-b: 2',3'-e][1,4] diazepin-6-one (nevirapine)](https://images-eureka.patsnap.com/patent_img/0360bf55-c604-4194-8700-18f50d0e9117/US20050059653A1-20050317-C00001.png)