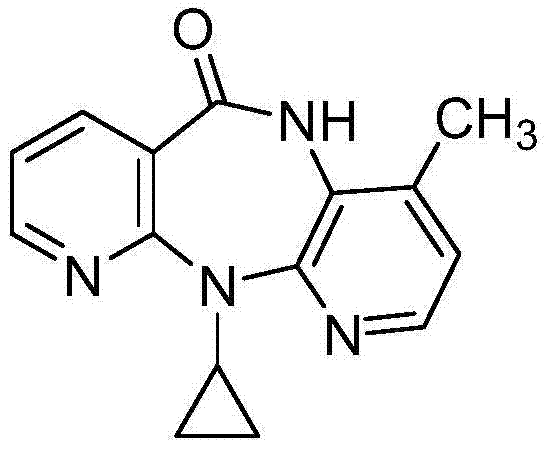
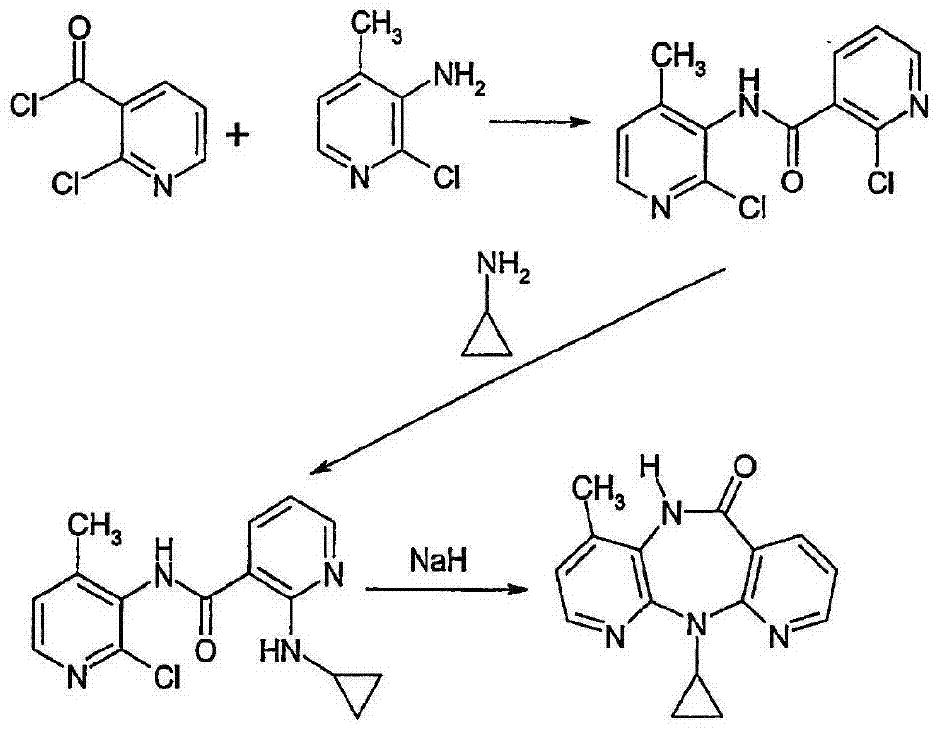
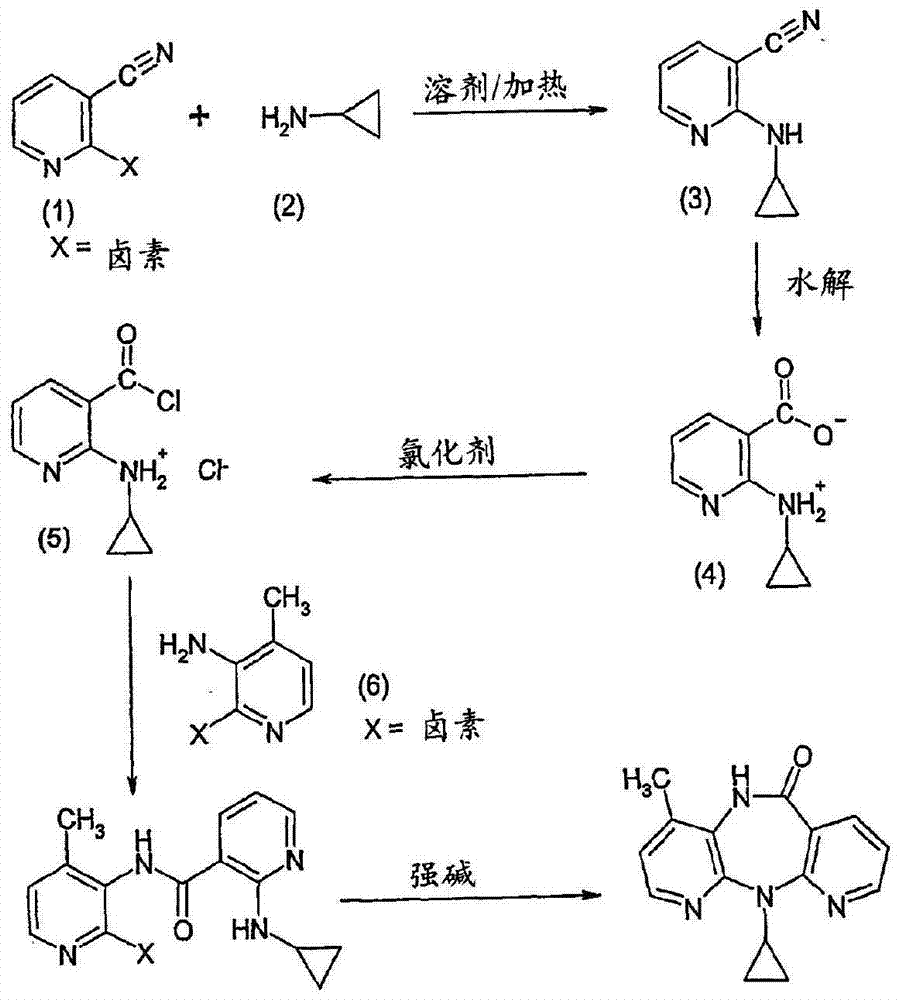
Methods for preparation of nevirapine and intermediate thereof
A technology of nevirapine and an intermediate, which is applied in the field of medicinal chemistry, can solve the problems of harsh process conditions, high production cost, unsuitable for industrialized production and the like, and achieves the effects of high purity, low cost and suitable for large-scale industrialized production
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] Embodiment 1 crude product preparation
[0043]Weigh compound (Ⅱ) (28.2g 0.1mol), calcium oxide (5.6g 0.1mol), cyclopropylamine (14.3g 0.25mol), KI (8.3g 0.05mol) and 50ml of diethylene glycol dimethyl ether were added to in the reaction vial. Heating in an oil bath to 80°C for reflux reaction, stirring for 12 hours, suction filtration after the reaction, collecting the filtrate, distilling off unreacted cyclopropylamine under reduced pressure, and adding 60% NaH (15.2g 0.38mol) under nitrogen protection Add to 100ml of diethylene glycol dimethyl ether, heat to 130°C, add dropwise the residue after the vacuum distillation, after dropping, continue to keep warm and stir for 1 hour, then evaporate the reaction solvent, add the residue to 150ml of water, stir Then add 65ml of cyclohexane, add glacial acetic acid to adjust the pH to 7-8, continue to stir for 1h and then filter. The filter cake is rinsed twice with ethanol and dried to obtain 20.1g of khaki crude nevirapine...
Embodiment 2
[0044] Embodiment 2 crude product preparation
[0045] Add compound (II) (28.2g 0.1mol), calcium oxide (5.6g 0.1mol), cyclopropylamine (14.3g 0.25mol), NaI (7.5g 0.05mol) and diglyme to the reaction flask in sequence 50ml. Heat the oil bath to 80°C for reflux reaction, stir for 12 hours, filter with suction after the reaction, collect the filtrate, evaporate unreacted cyclopropylamine under reduced pressure, and add 60% NaH (15.2g 0.38mol) under nitrogen protection Add to 100ml of diethylene glycol dimethyl ether, heat to 130°C, add dropwise the residue after the vacuum distillation, after dropping, continue to keep warm and stir for 1 hour, then evaporate the reaction solvent, add the residue to 150ml of water, stir Cyclohexane was added, acetic acid was added to adjust the pH to 7-8, stirring was continued for 1 h and then filtered. The filter cake was rinsed twice with ethanol and dried to obtain 19.8 g of khaki crude nevirapine, with a yield of 84.5% and a purity of 98.2%...
Embodiment 3
[0046] Embodiment 3 crude product preparation
[0047] Add compound (II) (28.2g 0.1mol), calcium oxide (5.6g 0.1mol), cyclopropylamine (14.3g0.25mol), KBr (6.0g 0.05mol) and 50ml of diethylene glycol dimethyl ether into the reaction flask . Heat the oil bath to 80°C for reflux reaction, stir for 12 hours, filter with suction after the reaction, collect the filtrate, evaporate unreacted cyclopropylamine under reduced pressure, and add 60% NaH (15.2g 0.38mol) under nitrogen protection Add to 100ml of diethylene glycol dimethyl ether, heat to 130°C, add dropwise the residue after the vacuum distillation, after dropping, continue to keep warm and stir for 1 hour, then evaporate the reaction solvent, add the residue to 150ml of water, stir Add 65 ml of cyclohexane, add acetic acid to adjust the pH to 7-8, continue to stir for 1 hour, filter, rinse the filter cake twice with ethanol, and dry to obtain 19.7 g of khaki-colored crude nevirapine, with a yield of 84.2% and a purity of 9...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com