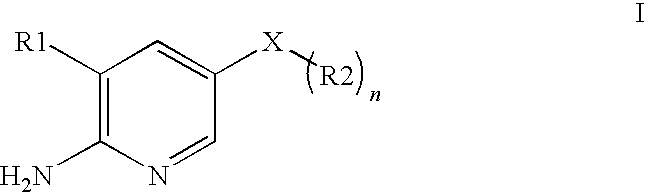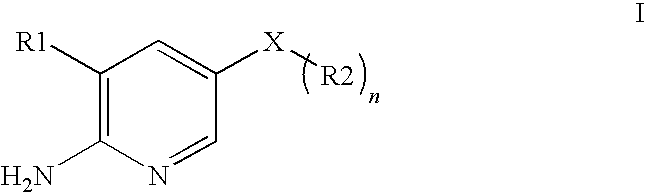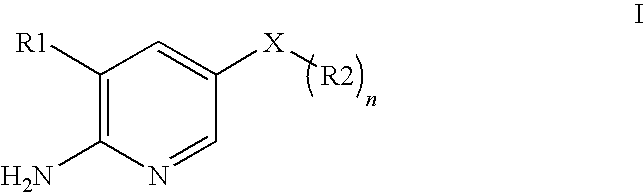Patents
Literature
850 results about "Aminopyridines" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Pyridines substituted in any position with an amino group. May be hydrogenated, but must retain at least one double bond.
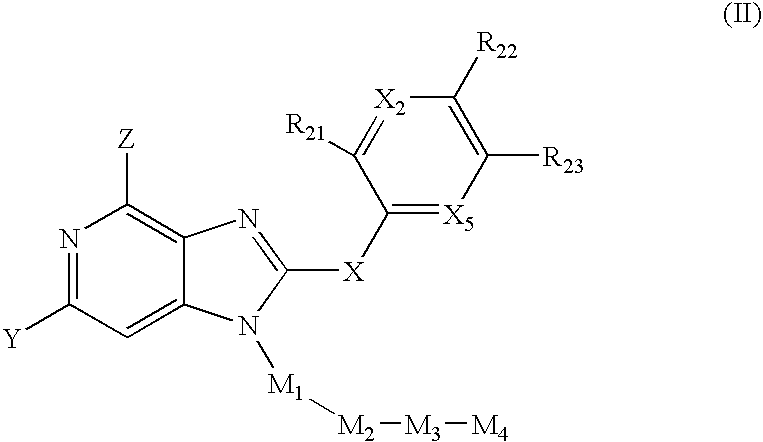
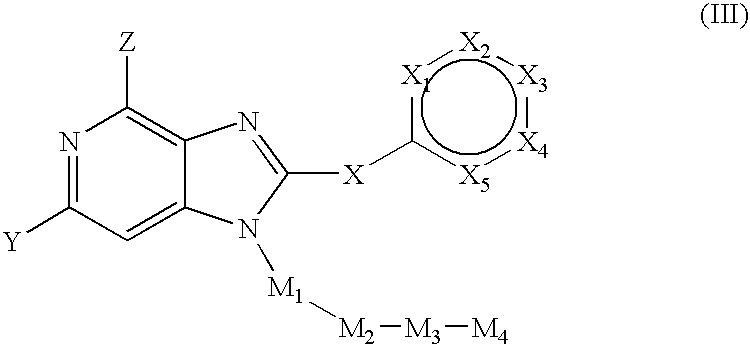
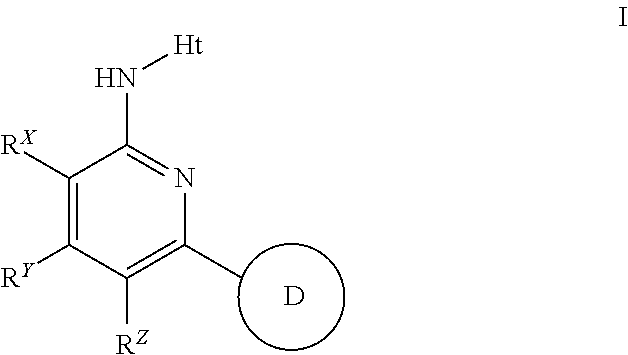
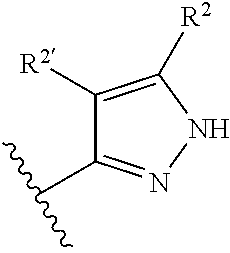
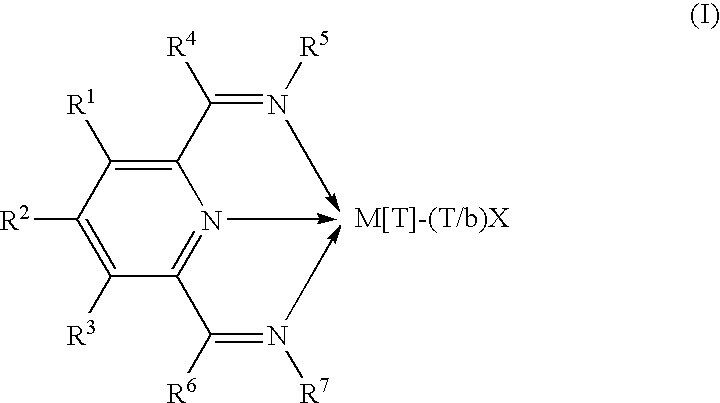
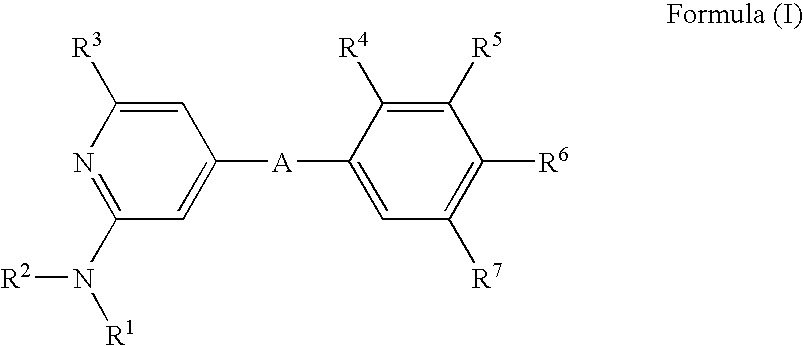
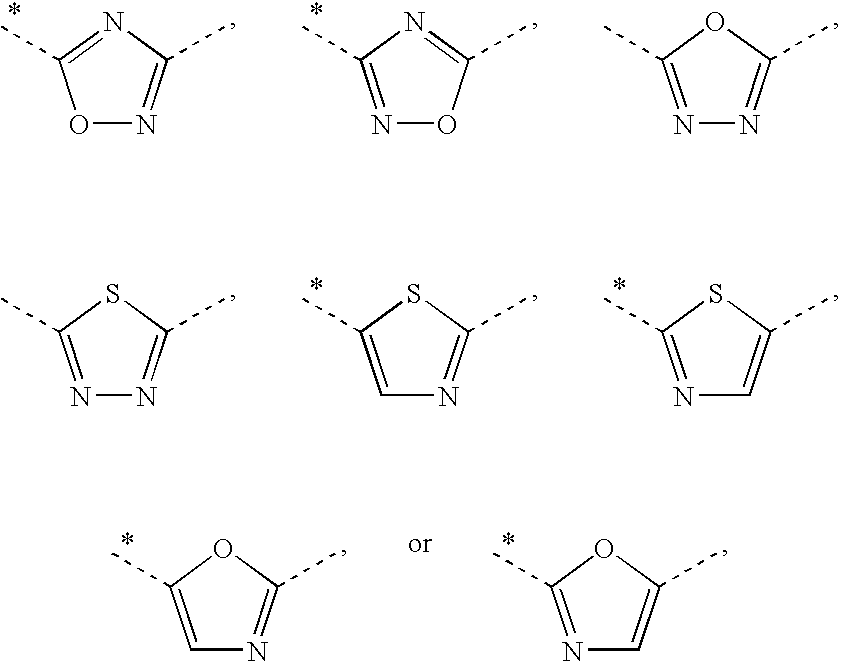
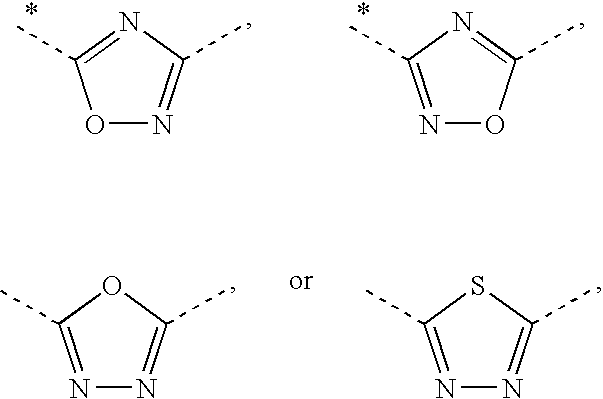
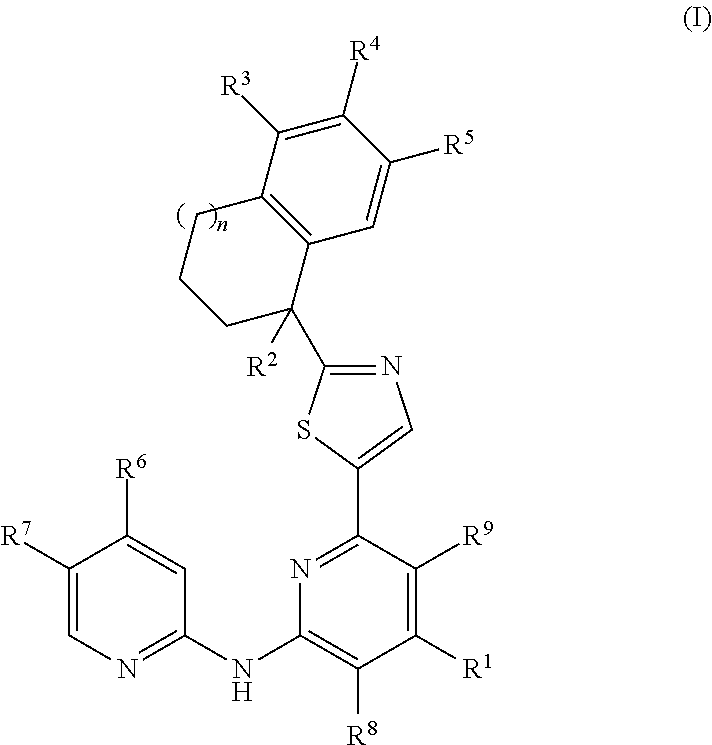
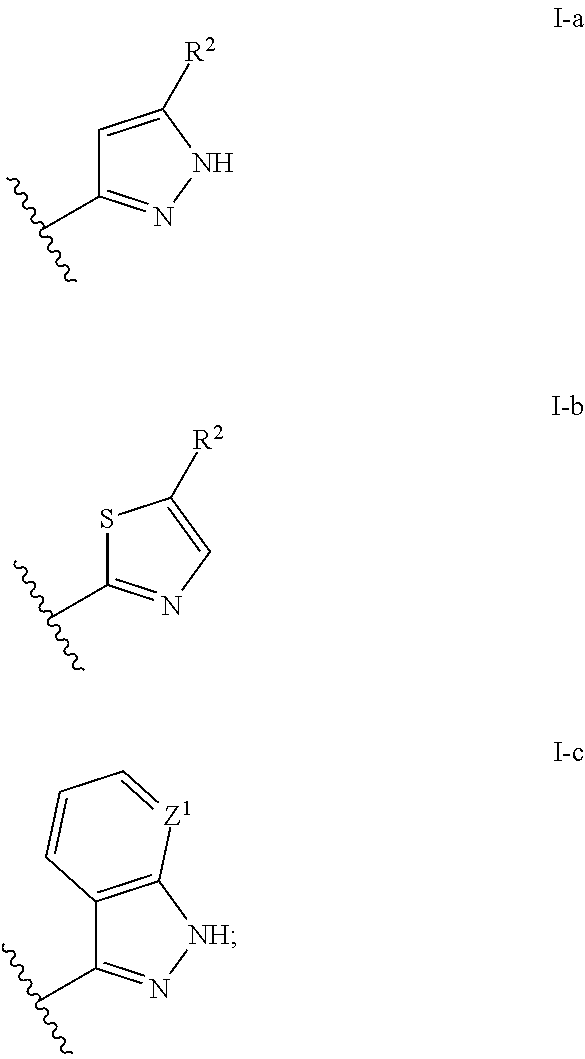
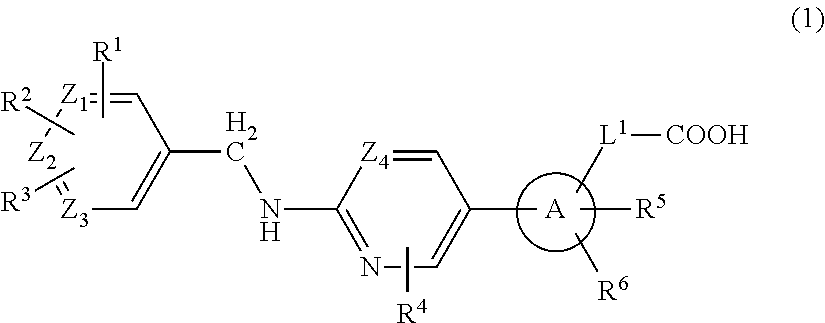
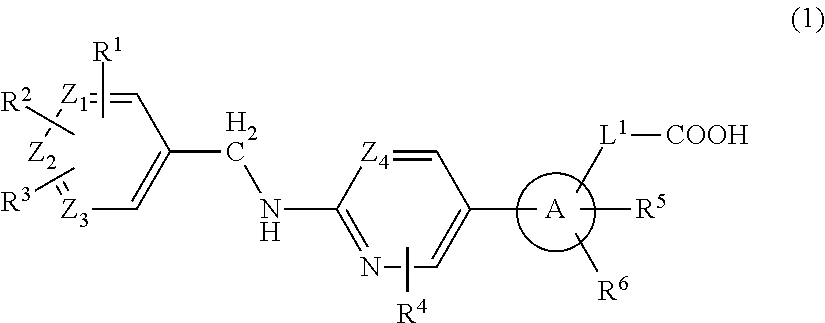
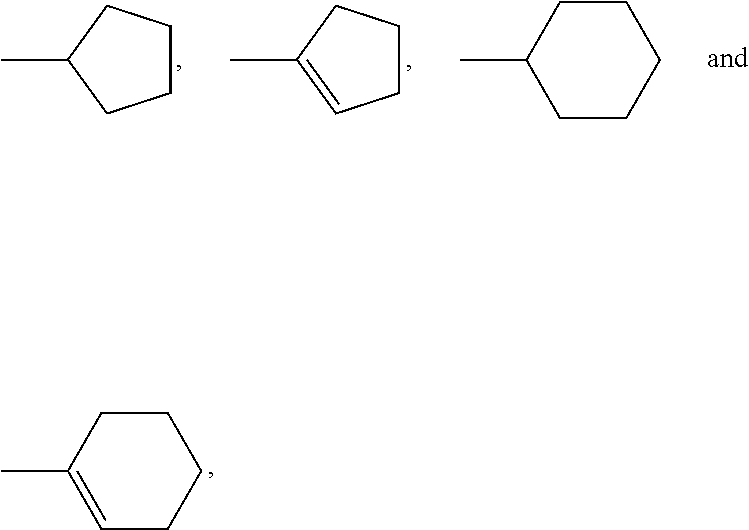
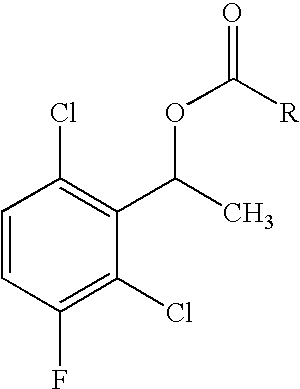
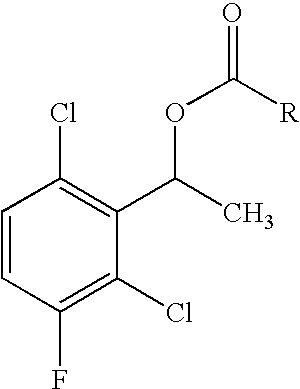
2-(Pyridin-2-ylamino)-pyrido[2,3-d]pyrimidin-7-ones
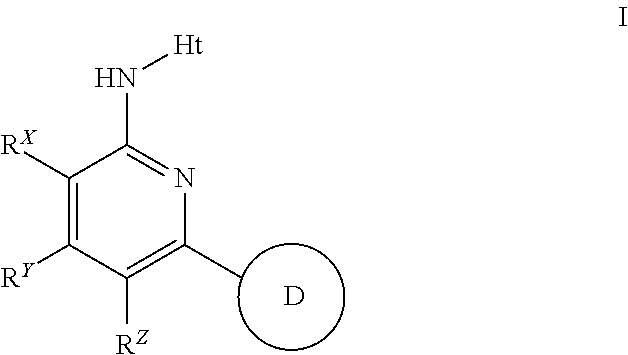
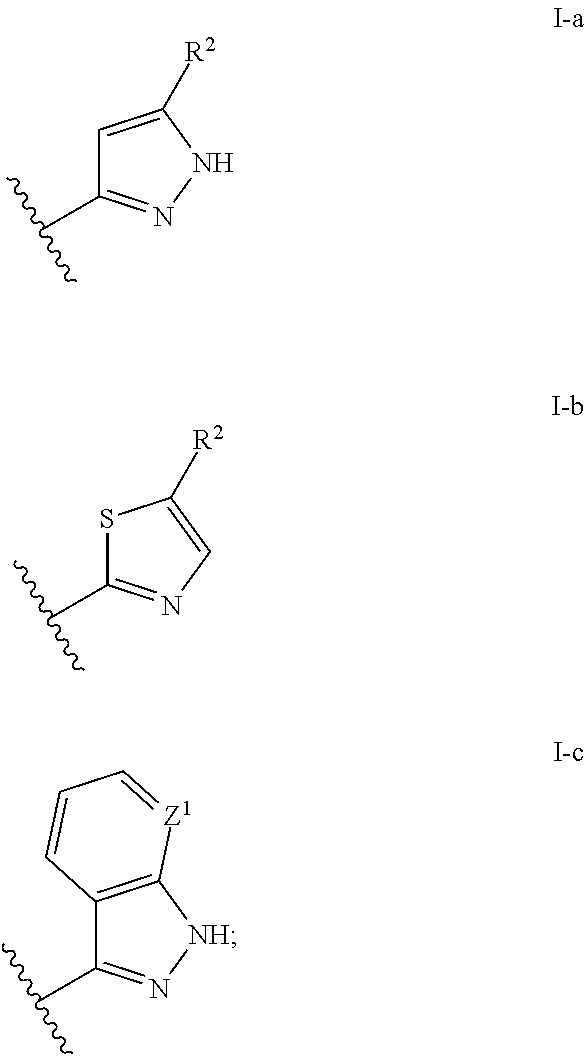
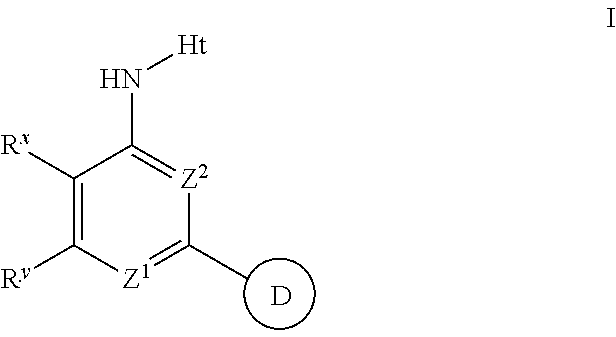
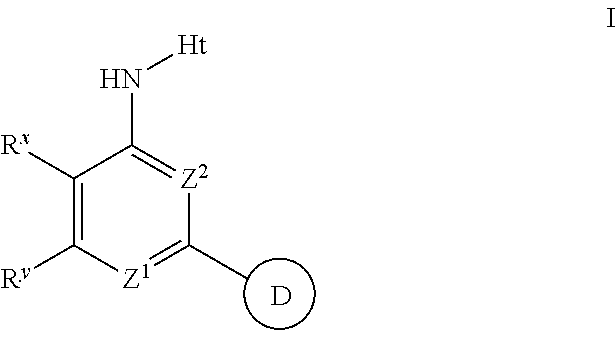
The present invention provides substituted 2-aminopyridines useful in treating cell proliferative disorders. The novel compounds of the present invention are potent inhibitors of cyclin-dependent kinases 4 (cdk4)
Owner:WARNER LAMBERT CO LLC
2-aminopyridine kinase inhibitors
InactiveUS20090197862A1Useful in treatmentReduce usageBiocideOrganic chemistryKinase inhibitionEnzyme inhibitor
2-Aminopyridine compounds having the structure of Formula I, and pharmaceutically acceptable salts of these compounds. Compounds of Formula I inhibit the activity of tyrosine kinase enzymes in animals, including humans, and are useful in the treatment and / or prevention of various diseases and conditions. In particular, compounds disclosed herein are inhibitors of kinases, in particular, but not limited to, KDR, Tie-2, Flt3, FGFR3, Ab1, Aurora A, c-Src, IGF-1R, ALK, c-MET, RON, PAK1, PAK2, and TAK1, and can be used in the treatment of proliferative diseases, such as, but not limited to, cancer. The present invention is also directed to a pharmaceutical composition comprising a therapeutically effective amount of a compound of Formula I, or a pharmaceutically acceptable salt thereof, and a pharmaceutically acceptable carrier. The present invention is further directed to a method of treating a patient having a condition which is mediated by protein kinase activity by administering to the patient a therapeutically effective amount of the above-mentioned pharmaceutical composition.
Owner:OSI PHARMA LLC
Fused amino pyridine as hsp90 inhibitors
The present invention relates to HSP90 inhibitors containing fused amino pyridine core that are useful as inhibitors of HSP90 and their use in the treatment of HSP90 related diseases and disorders such as cancer, an autoimmune disease, or a neurodegenerative disease.
Owner:CURIS INC
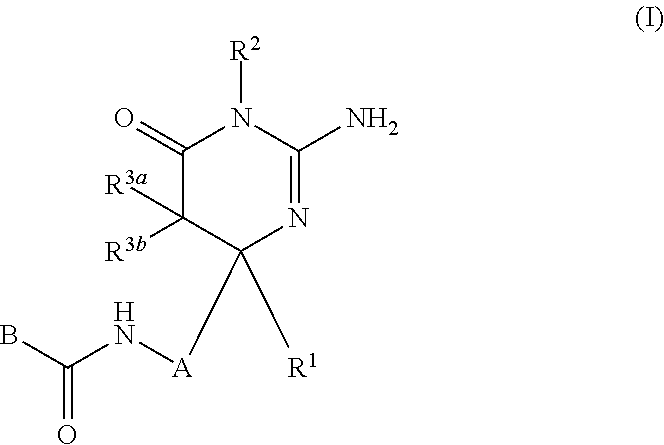
2-aminopyrimidin-4-one and 2-aminopyridine derivatives both having bace1-inhibiting activity
InactiveUS20110237576A1Inhibit productionUseful in therapyBiocideNervous disorderDiseaseChemical compound
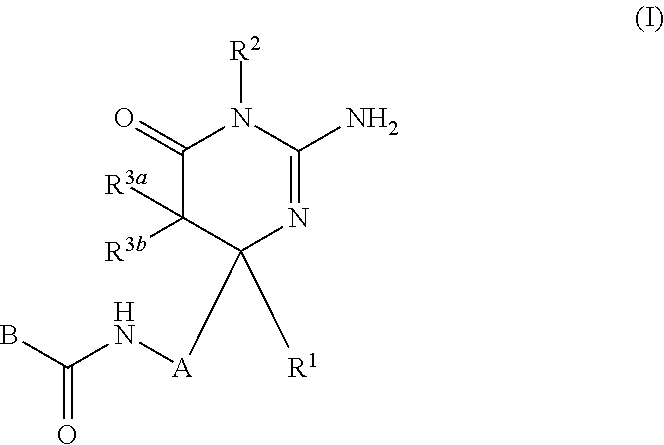
The present invention provides a compound which has an effect of inhibiting amyloid-β production and is useful as a therapeutic agent for diseases induced by production, secretion and / or deposition of amyloid-β proteins. The present invention relates to a compound represented by formula (I) or a pharmaceutically acceptable salt or solvate thereof:wherein A is optionally substituted carbocyclic diyl or optionally substituted heterocyclic diyl; B is an optionally substituted carbocyclic group or an optionally substituted heterocyclic group; R1 is a group such as optionally substituted lower alkyl; R2 is a group such as hydrogen; and R3a and R3b are each independently a group such as hydrogen, provided that the following compound is excluded.
Owner:SHIONOGI & CO LTD
Synergistic herbicidal compositions containing aminopyralid, 2,4-dichlorophenoxyacetic acid and atrazine
Owner:CORTEVA AGRISCIENCE LLC
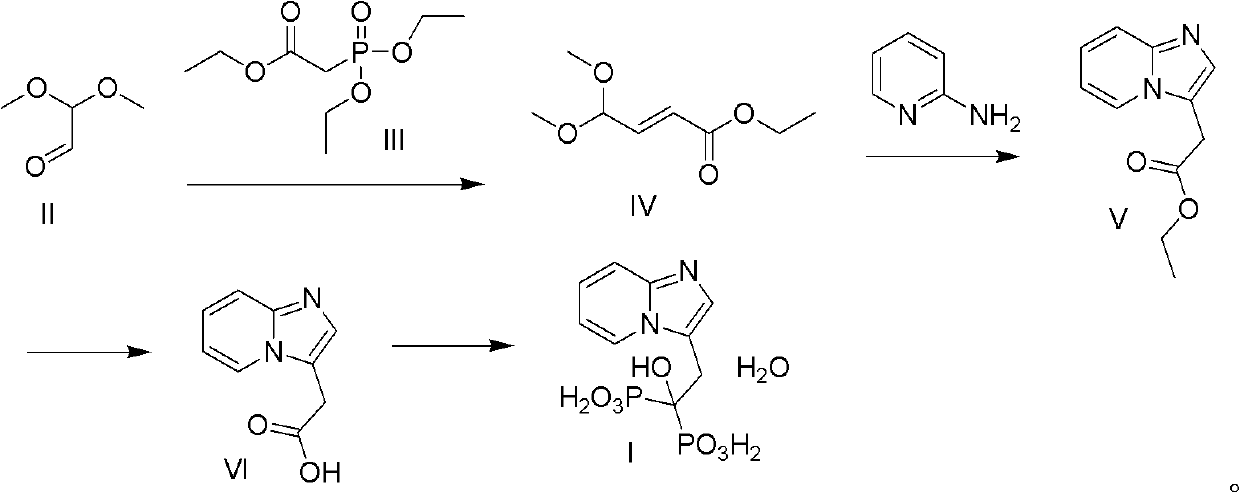
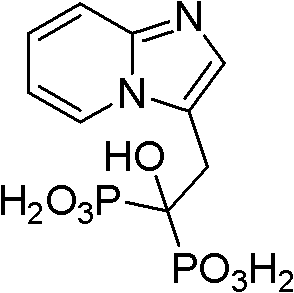
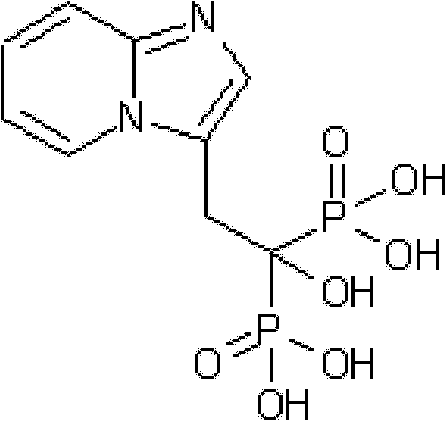
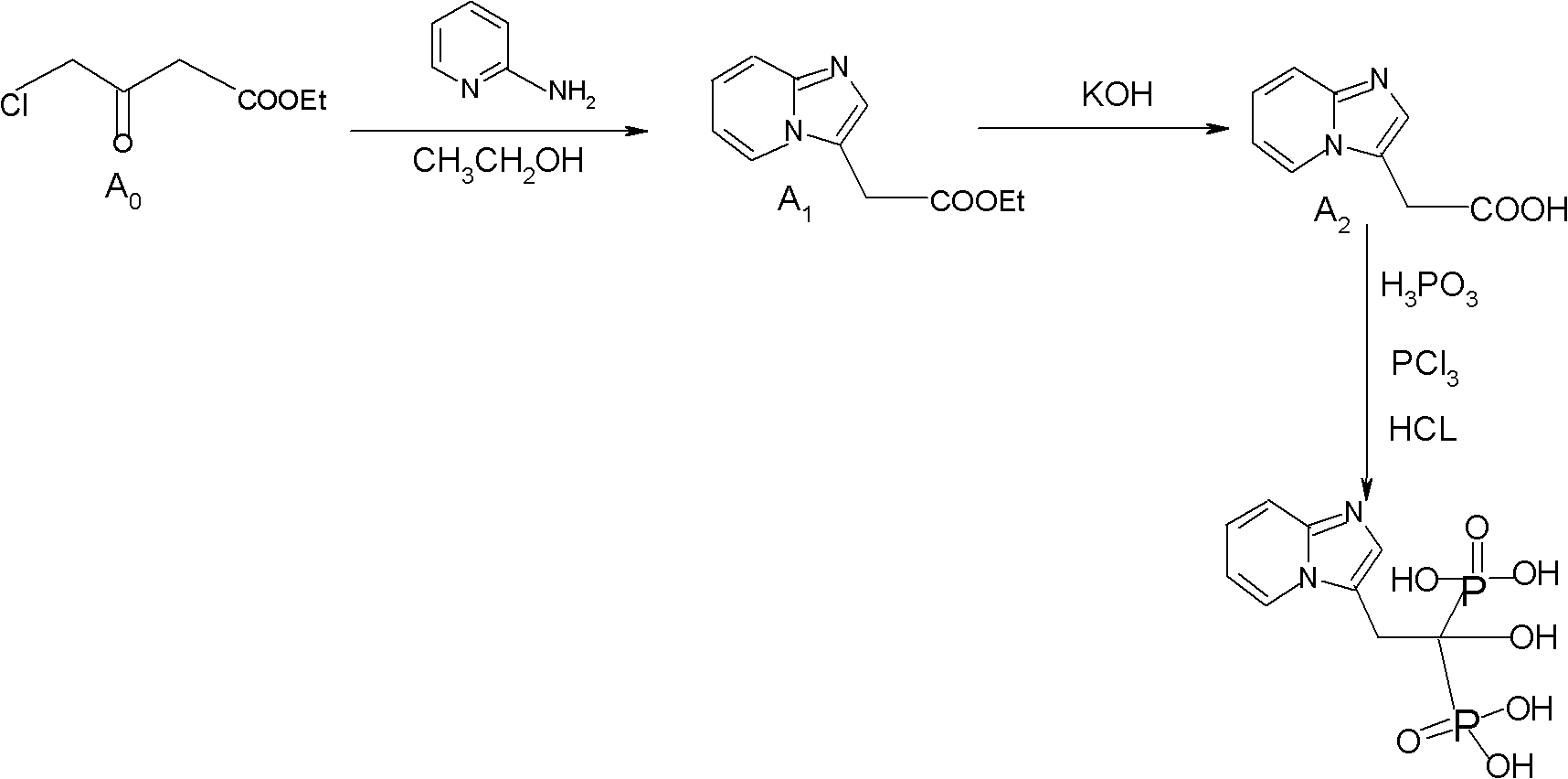
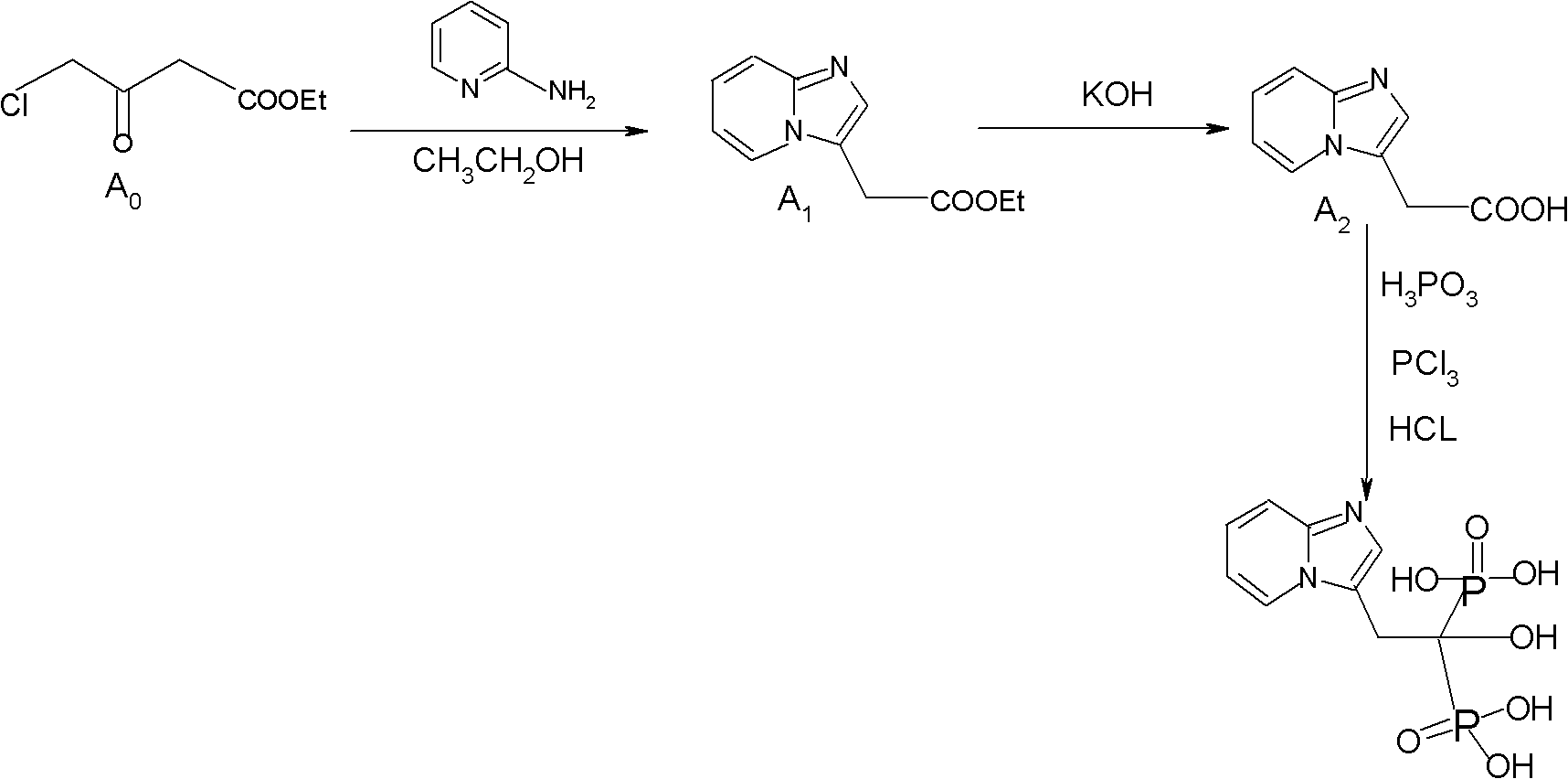
Method for preparing minodronate
ActiveCN102020676AThe reaction conditions are mild and controllableShort reaction stepsGroup 5/15 element organic compoundsBromineSodium cyanide
The invention discloses a method for preparing minodronate, which comprises the following steps of: condensing a compound II and a compound III to form a compound IV, closing rings of the compound IV and 2-aminopyridine to obtain a compound V, hydrolyzing the compound V into a compound VI, and finally performing phosphonation to form a compound I. The method avoids using virulent chemical reagents such as sodium cyanide or bromine and the like, has mild and controllable reaction condition, short reaction step, high yield and low cost, and is suitable for industrialized production.
Owner:JIANGSU LEEWAY BIOLOGICAL TECH
Method for catalyzing dynamic kinetic resolution of arylamine via racemization catalyst
InactiveCN102533922AGood stability for repeated useMild reaction conditionsOrganic chemistry methodsChemical recyclingChlorobenzenePtru catalyst
The invention discloses a method for catalyzing dynamic kinetic resolution of arylamine via a racemization catalyst, comprising the following steps of: 1) adding p-chlorophenol, n-pentanoic acid, dicyclohexylcarbodiimide and 4-dimethylamino-pyridine, and carrying out mixing, filtration, drying, concentration and column chromatography to obtain a pentanoic acid p-chlorophenyl ester acyl donor; 2) carrying out coprecipitation on magnesium chloride solution and aluminum chloride solution and carrying out water-heat treatment to obtain chloridion intercalated hydrotalcite, adding the chloridion intercalated hydrotalcite in lauryl sodium sulfate aqueous solution, and carrying out backflow, cooling, centrifugation, water washing, acetone washing and drying to obtain a carrier; 3) adding palladium salt and the carrier, and carrying out heating, ascorbic acid addition, centrifugation, water washing, acetone washing and freeze-drying to obtain the racemization catalyst; and 4) adding arylamine, the acyl donor, lipase and the racemization catalyst in toluene and placing in a stainless steel reactor to add hydrogen so as to obtain amide. The method provided by the invention is used for catalyzing the dynamic kinetic resolution of arylamine, has rapid reaction rate, low temperature, high conversion rate and high product optical purity, and has great application value.
Owner:ZHEJIANG UNIV
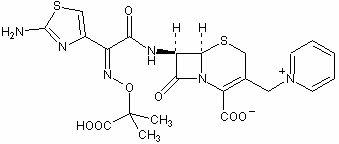
Synthetic methods of ceftazidime intermediate and ceftazidime
The invention relates to a synthetic method of ceftazidime intermediate; 7-amino cephalsporanic acid is used as a raw material; a silanization reaction, an iodination reaction, and a pyridine reaction are performed; the obtained product is added with an oxidant, and hydrochloric acid or is added into a mixed solvent of an organic solvent and water to prepare a halogen acid salt of the ceftazidimeintermediate (6R, 7R)-7-amino-3-pyridine methyl-ceph-3-ene-4-carboxylic acid. The invention also provides a method for preparing ceftazidime by using the obtained intermediate halogen acid salt. The ceftazidime intermediate and ceftazidime prepared by the methods have high yield, and low production cost; the operation is simple; the discharge of three wastes is less; treatment and recovery are easy, and the methods are applicable to industrial production.
Owner:QILU ANTIBIOTICS PHARMA
Preparation method of two-dimension nitrogen- and phosphorus-doped graphene
The invention discloses a preparation method of two-dimension nitrogen- and phosphorus-doped graphene. The preparation method particularly includes following steps: adding hydrotalcite to a proper amount of a sodium butyrate solution, stirring the mixture, aging the mixture, adding 4-dimethyl aminopyridine and n-butyl phosphate, stirring the mixture in a constant-temperature water bath at 60-70 DEG C for 6-8 h, separating a precipitation from liquid, washing the precipitation with deionized water for 2-3 times, drying and grinding the precipitation, sieving the precipitation through a sieve being 20-40 in meshes to obtain modified hydrotalcite powder, heating the modified hydrotalcite powder in a vacuum tubular furnace to 400-600 DEG C under a vacuum condition for calcining the powder for 2-4 h, adding the calcined powder to a hydrochloric acid solution, separating the precipitation and finally heating the precipitation to 2000-2500 DEG C under the vacuum condition to perform heat treatment for 3-6 h, and cooling a product to obtain the two-dimension nitrogen- and phosphorus-doped graphene. The preparation method is simple in raw materials and is mild in conditions.
Owner:JINING XINRUIDA INFORMATION TECH CO LTD
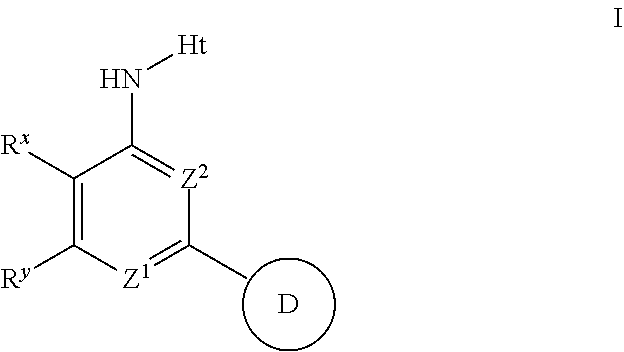
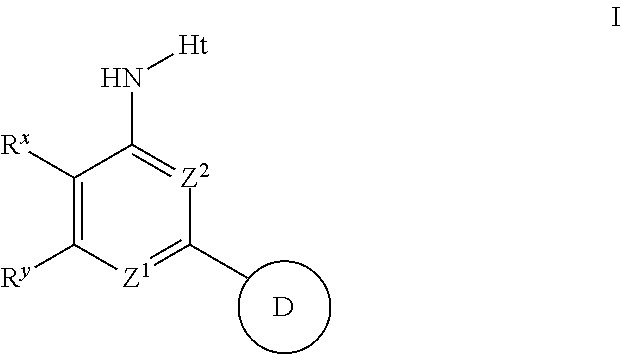
Aminopyridines and aminopyrimidines useful as inhibitors of protein kinases
The present invention relates to compounds useful as inhibitors of protein kinase. The invention also provides pharmaceutically acceptable compositions comprising said compounds and methods of using the compositions in the treatment of various disease, conditions, or disorders. The invention also provides processes for preparing compounds of the inventions.
Owner:VERTEX PHARMA INC
A kind of 6-aminopyridine-3-carboxylic acid chelating resin and preparation method thereof
ActiveCN102295723AHigh stability constantGood choiceOther chemical processesPolymer scienceChloromethyl Ether
The invention discloses a 6-aminopyridine-3-carboxylic acid chelating resin and its preparation method, which belongs to the field of chelating resin. The resin has a following structural unit: a functional group is 6-aminopyridine-3-carboxylic acid, wherein the color is yellowish, the particle size is 0.45-0.6 mm, the content of the functional group is 1.37-2.38 mmol / g. The preparation method comprises the following steps: using styrene as a monomer, employing a suspension polymerization method to prepare a low crosslinking degree macroporous styrene-divinylbenzens copolymer, air-flow dryingto obtain low crosslinking macroporous polystyrene-divinylbenzens resin (white ball for short); immersing the white ball in chloromethyl ether, adding zinc chloride as a catalyst, carrying out a chloromethylation reaction to obtain chloromethylated low crosslinking polystyrene-divinylbenzens resin (chlorine ball for short); taking N,N-dimethyl formamide as a swelling agent, taking DMF as a swelling agent to swell the chlorine ball, dissolving 6-aminopyridine-3-carboxylic acid and sodium carbonate in N, N-dimethyl formamide for reaction, then adding the above swelled chlorine ball, stirring for reacting to prepare 6-aminopyridine-3-carboxylic acid chelating resin. The resin prepared by the invention is suitable for selectively absorbing and separating heavy metal ions of copper and the like.
Owner:NANJING UNIV
Aminopyridines useful as inhibitors of protein kinases
The present invention relates to compounds useful as inhibitors of protein kinases. The invention also provides pharmaceutically acceptable compositions comprising those compounds, the methods of using the aforementioned compounds, and compounds in the treatment of various diseases, conditions, and disorders. The invention also provides processes for preparing compounds of the invention.
Owner:VERTEX PHARMA INC
Aminopyridines and aminopyrimidines useful as inhibitors of protein kinases
The present invention relates to compounds useful as inhibitors of protein kinase. The invention also provides pharmaceutically acceptable compositions comprising said compounds and methods of using the compositions in the treatment of various disease, conditions, or disorders. The invention also provides processes for preparing compounds of the inventions.
Owner:VERTEX PHARMA INC
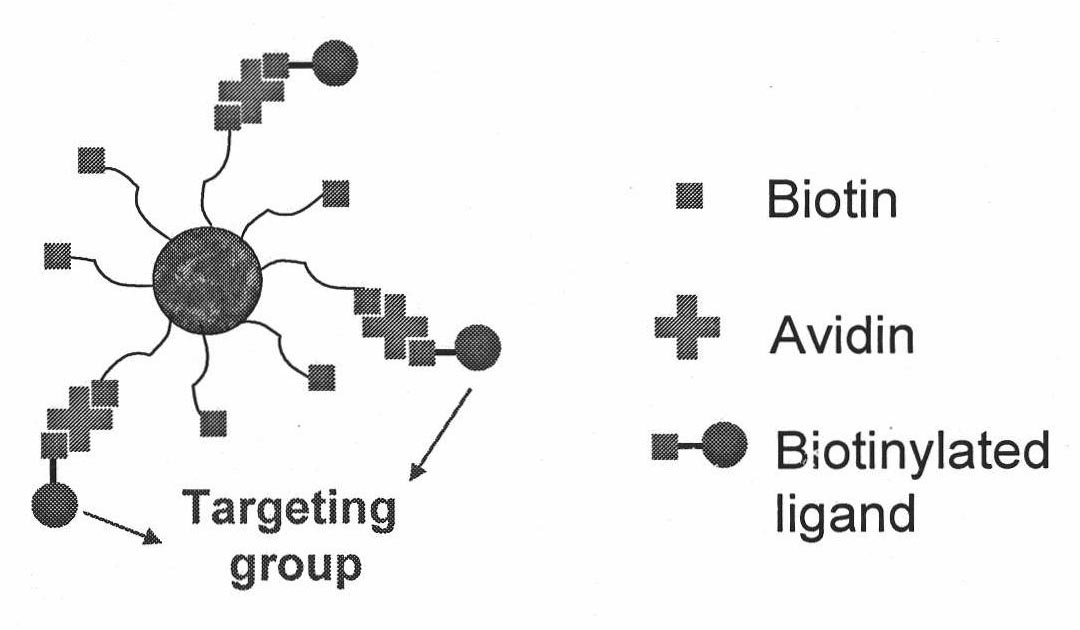
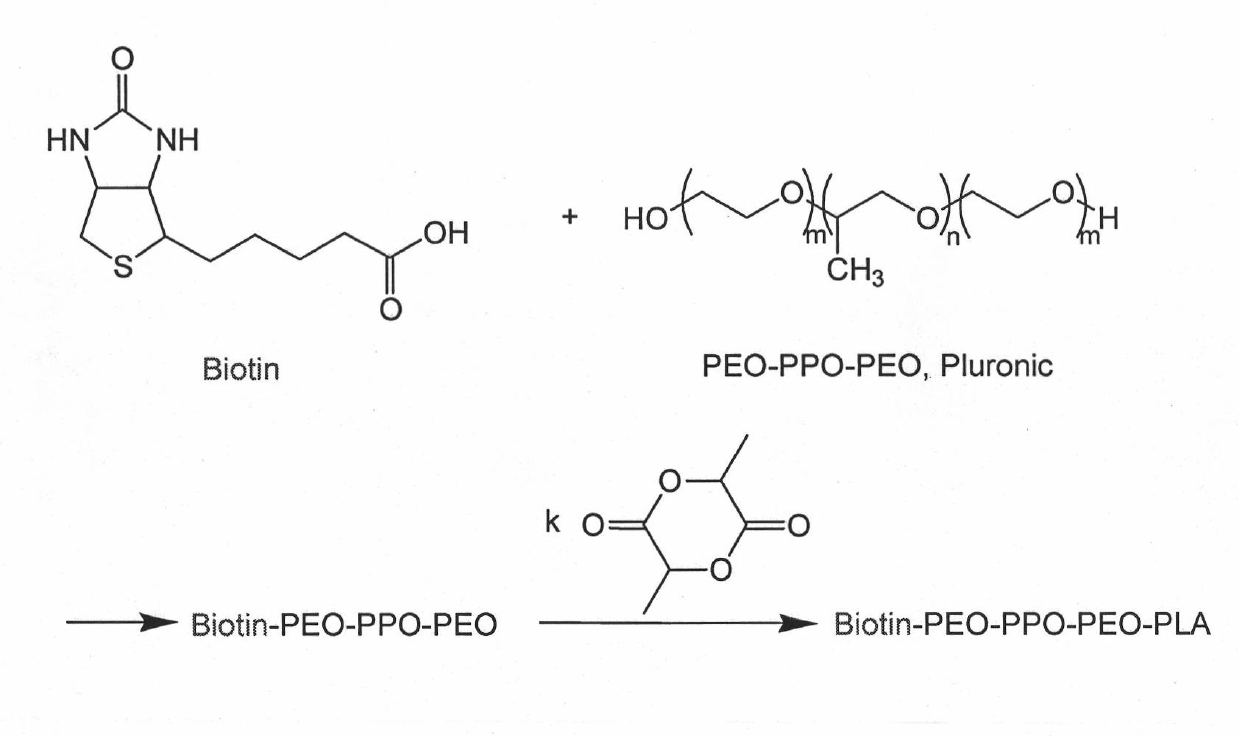
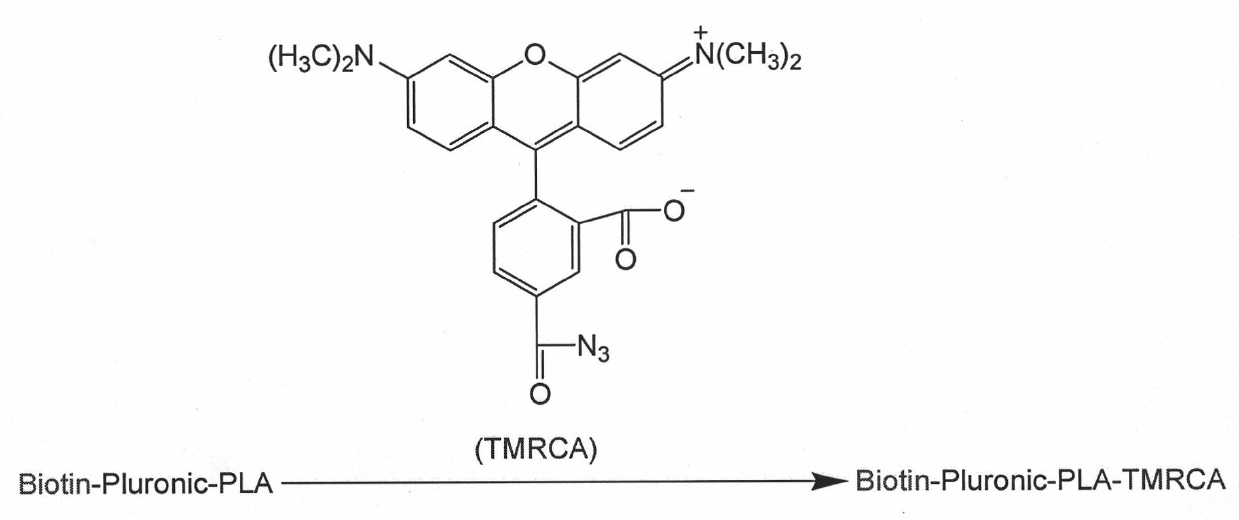
Targeted polymer medicament carrier and preparation method and application thereof
InactiveCN102000340AOvercome expensiveMultiple choicePharmaceutical non-active ingredientsIn-vivo testing preparationsWater bathsIce water
The invention relates to a targeted polymer medicament carrier and a preparation method and application thereof. The targeted polymer medicament carrier has a molecular structural formula shown in the graph. The preparation method comprises the following steps of: a) feeding Pluronic and Biotin in a molar ratio of 1:1.1-1.5; dissolving the mixture in dichloromethane; adding 4-dimethylamino pyridine; dropwise adding 1,3-dicyclohexyl carbodiimide in an ice water bath; reacting at room temperature for 24 to 48 hours; extracting reactive fluid with 10 to 15 percent NaHCO3; freezing over night; filtering to remove undissolved substances; concentrating the reactive fluid; dropping the reactive fluid into cold absolute ethyl ether; filtering, and drying in vacuum; and b) dissolving a product with dry toluene, and distilling the product in the presence of argon gas; dehydrating by an azeotropy method; cooling to the room temperature; adding lactide according to 50 to 90 percent of the weight of the product in the presence of the argon gas, and adding stannous octoate according to 0.1 to 0.15 percent of the weight of the lactide; heating to the temperature of between 120 and 140 DEG C; reacting for 6 to 8 hours under stirring; immersing a reactant into the cold ethyl ether, and filtering; and dissolving polymer by using dichloromethane, immersing into methanol, and filtering and drying. The carrier can be used as carriers of medicaments for treating and diagnosing cancers.
Owner:JIANGXI SCI & TECH NORMAL UNIV
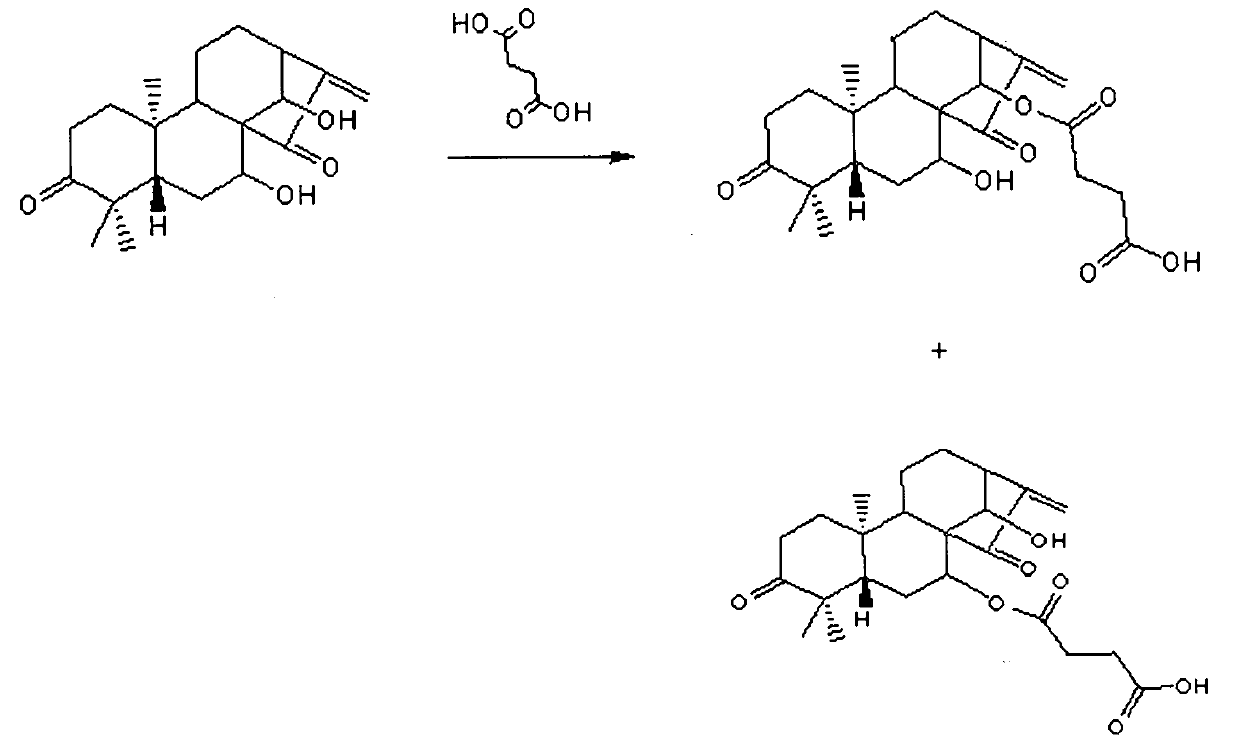
Glaucocalyxin A acid ester derivative as well as preparation method and application of Glaucocalyxin A acid ester derivative
InactiveCN101993370AGood inhibitory effectOrganic compound preparationCarboxylic acid esters preparationPhosphate4-Dimethylaminopyridine
The invention discloses a structure of a Glaucocalyxin A acid ester derivative as well as a preparation method and an application of the Glaucocalyxin A acid ester derivative in treating cancers. The molecular structure of the Glaucocalyxin A acid ester derivative has a general formula as shown in the specification, wherein R1 and R2 are succinic acid substituent-C4H5O4, glutaric acid substituent-C5H7O4M, glycine derivative substituent-C6H8NO5 of succinic acid, and glycine substituent-C7H10NO5 or glycine substituent-OH of glutaric acid. The preparation method has the following steps: in the presence of tetrahydrofuran and 4-dimethylamino pyridine, the extracted Glaucocalyxin A and succinic acid or glutaric acid are reacted to obtain the succinic acid ester or glutaric acid ester of the Glaucocalyxin A disclosed in the invention, and the Glaucocalyxin A acid ester derivative reacts with N-Hydroxysuccinimide and glycine in turn in the presence of a buffer solution of tetrahydrofuran and phosphate to obtain the derivative of the acid ester of the Glaucocalyxin A and the glycine disclosed in the invention.
Owner:SHANGHAI JINHAO PHARMA DEV
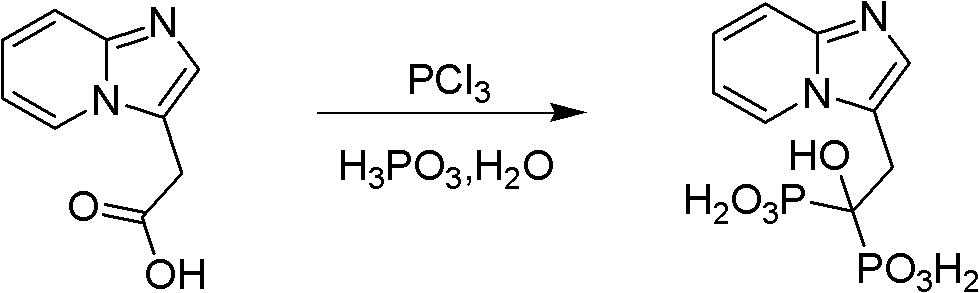
Synthesis method of minodronate midbody and synthesis of minodronate
ActiveCN102153585AAvoid pollutionImprove securityGroup 5/15 element organic compoundsSynthesis methodsFiltration
The invention relates to the field of pharmaceutical chemistry, in particular to a synthesis method of a minodronate midbody and synthesis of minodronate. The preparation method of minodronate includes the following steps: using organic solvent to dissolve 2-aminopyridine, adding 4-acetyl chloride ethyl acetoacetate for reaction, monitoring the reaction solution by TLC(Thin-Layer Chromatography) until spots of 4-acetyl chloride ethyl acetoacetate disappear, concentrating to a dry state, dissolving concentrate in water, washing a water layer to remove impurities, extracting the water layer with the organic solvent, washing extract liquor, separating out an organic layer, conducting filtration, and concentrating the concentrate to be in a dry state, thereby obtaining A1.
Owner:福建太平洋制药有限公司
Olefin polymerization catalysts, their synthesis and use
InactiveUS7541413B2Molecular sieve catalystsOrganic-compounds/hydrides/coordination-complexes catalystsPolymer sciencePtru catalyst
A catalyst system for the polymerization or copolymerization of α-olefins comprises a bis(imino)pyridyl complex of a transition metal on a support having an aluminum halide and / or an alkylaluminum halide chemically or physically bonded thereto.
Owner:EXXONMOBIL CHEM PAT INC
Amino-pyridine derivatives as s1p1 /edg1 receptor agonists
InactiveUS20100087417A1Reduction tendencyEffective activityBiocideSenses disorderImmunomodulating AgentAgonist
The invention relates to novel amino-pyridine derivatives, their preparation and their use as pharmaceutically active compounds. Said compounds particularly act as immunomodulating agents.
Owner:ACTELION PHARM LTD
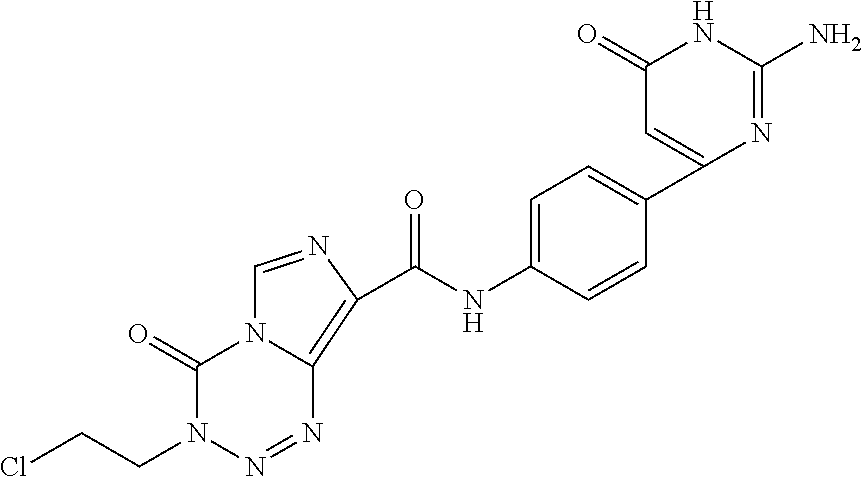
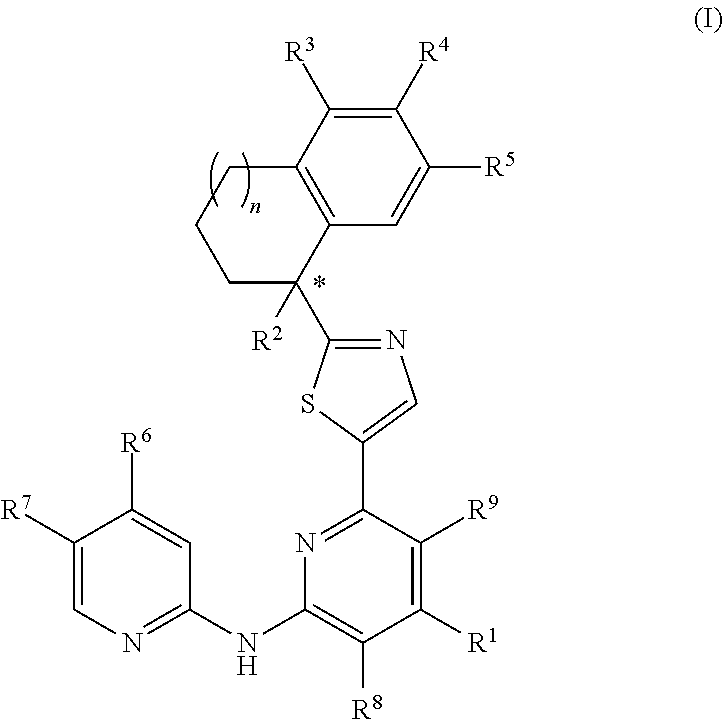
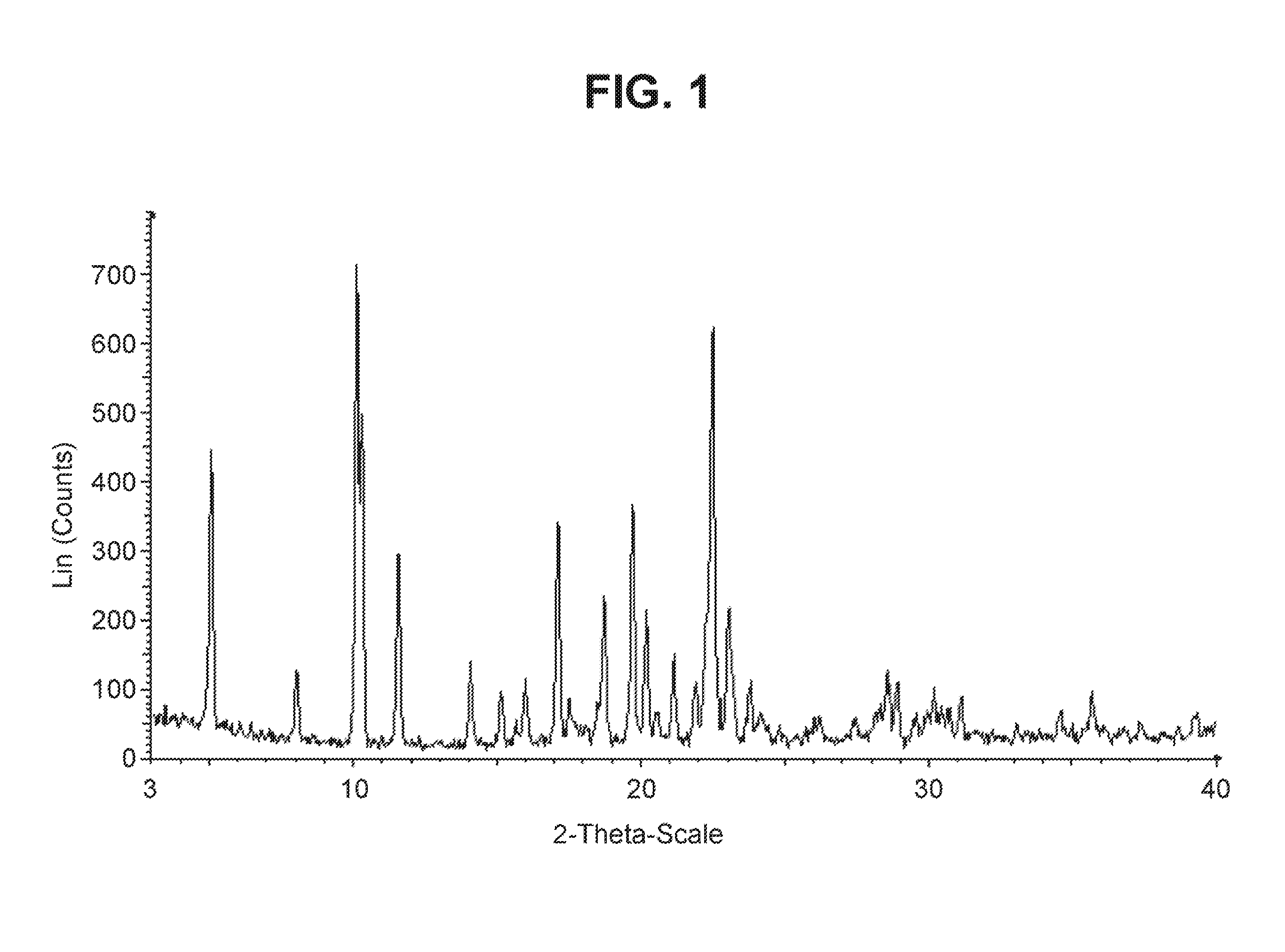
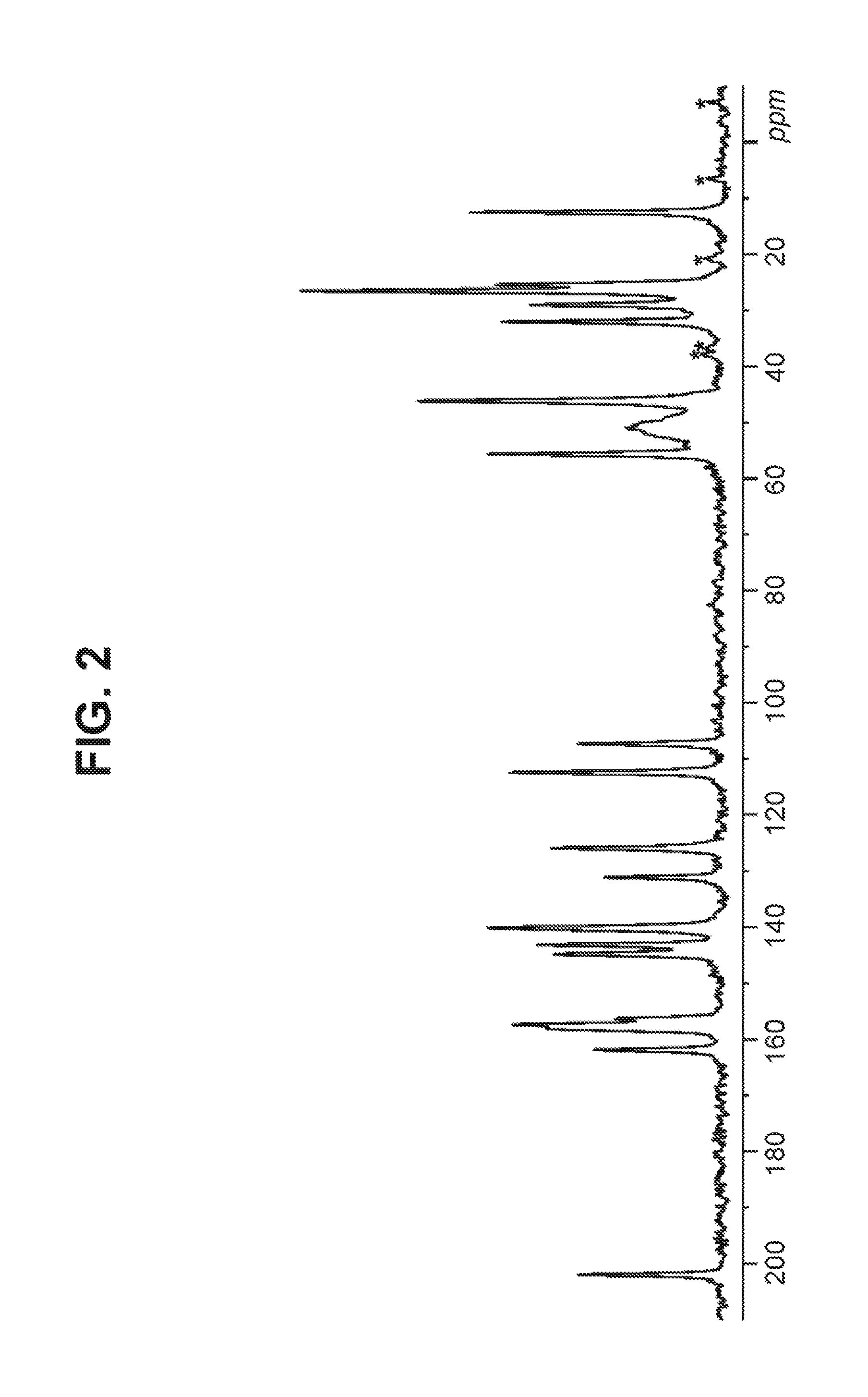
Crystal form of benzoylaminopyridine derivative, and application thereof
ActiveCN110922407AGood treatment effectEffective treatmentOrganic active ingredientsNervous disorderDiseasePharmaceutical drug
The invention relates to a crystal form of a benzoylaminopyridine derivative, and an application of the crystal form, and also relates to a pharmaceutical composition containing the crystal form, andan application of the crystal form or the pharmaceutical composition in the preparation of drugs for preventing, treating or alleviating ASK1-regulated diseases of patients.
Owner:SUNSHINE LAKE PHARM CO LTD
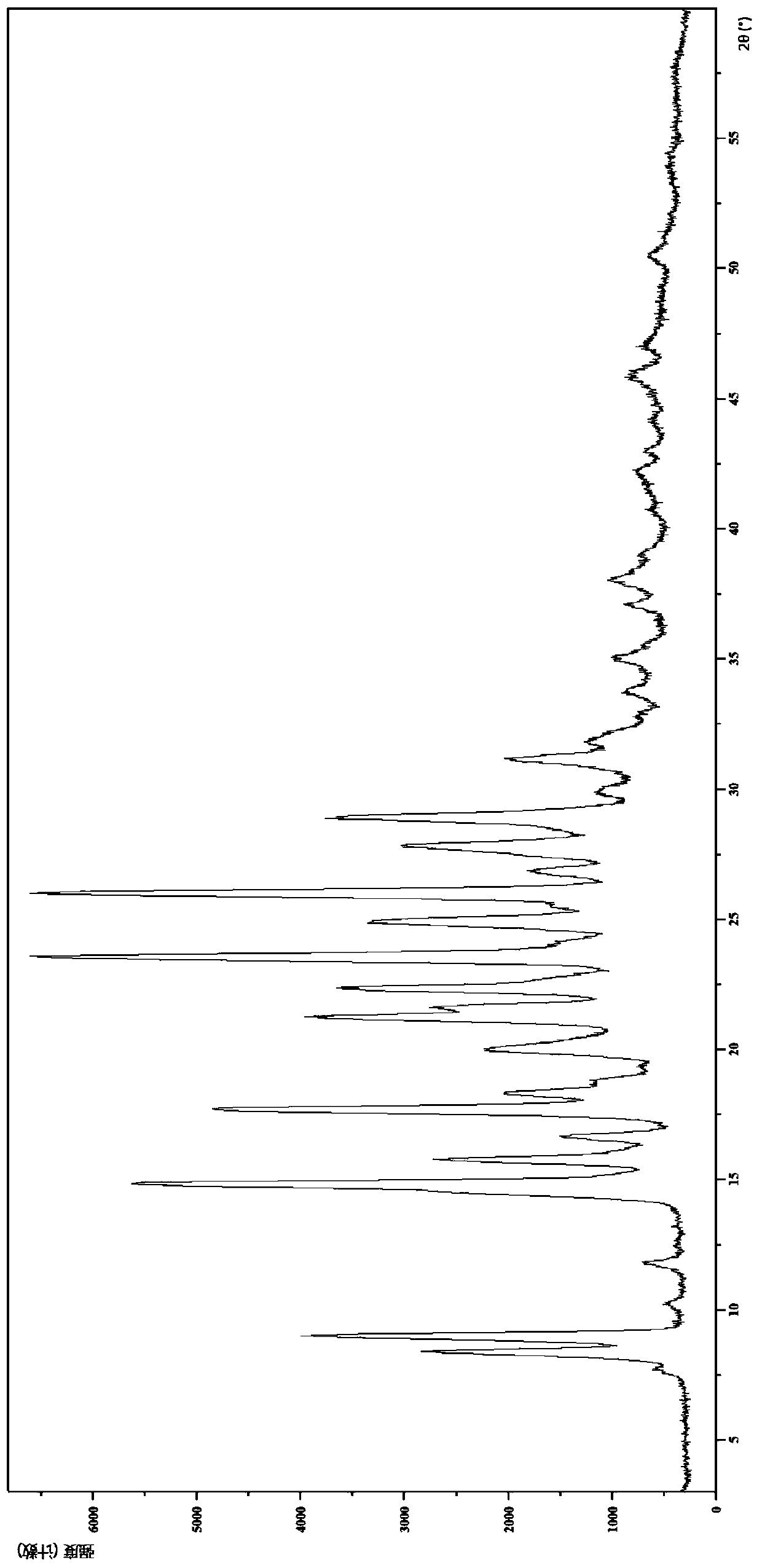
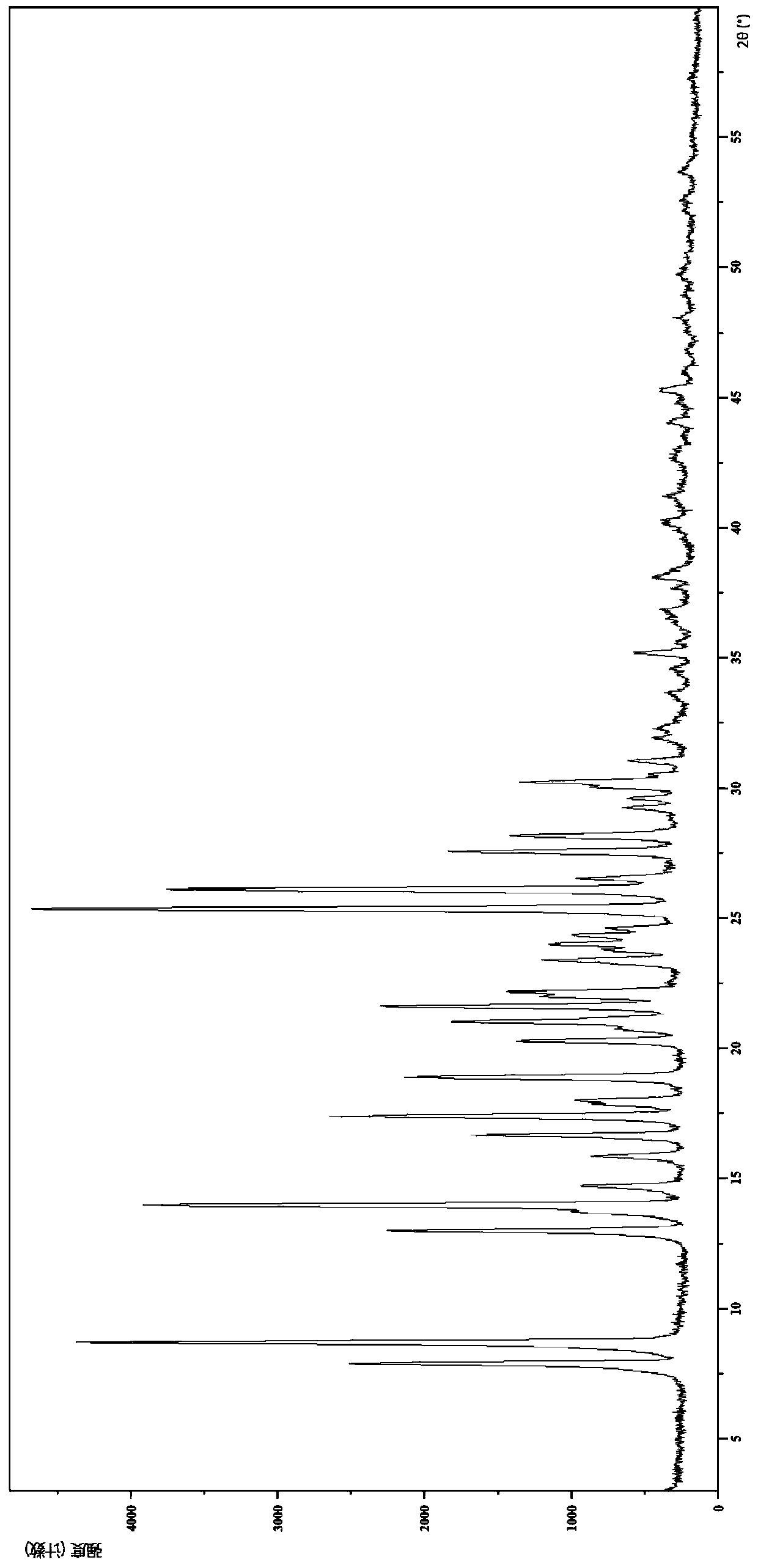
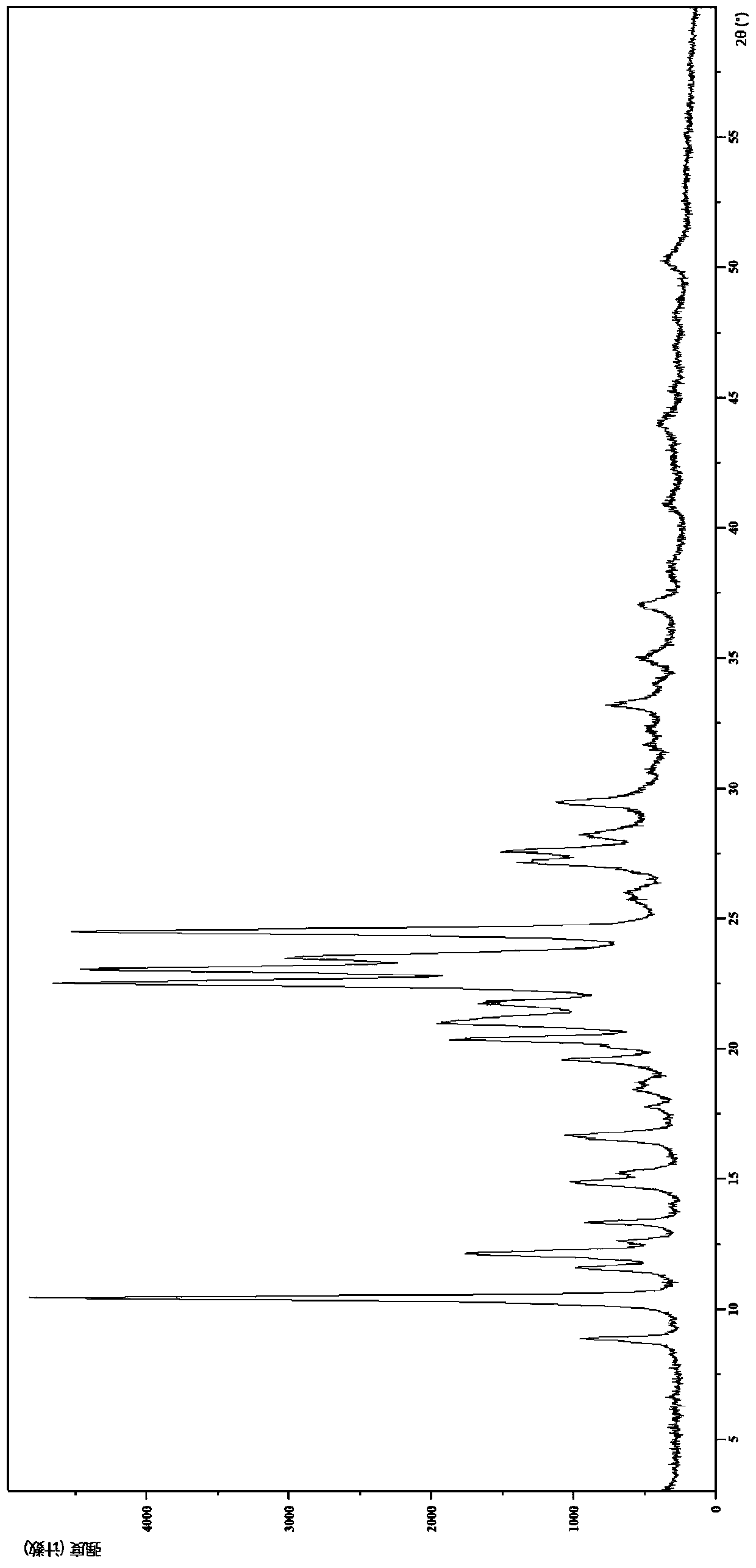
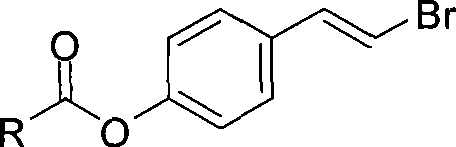
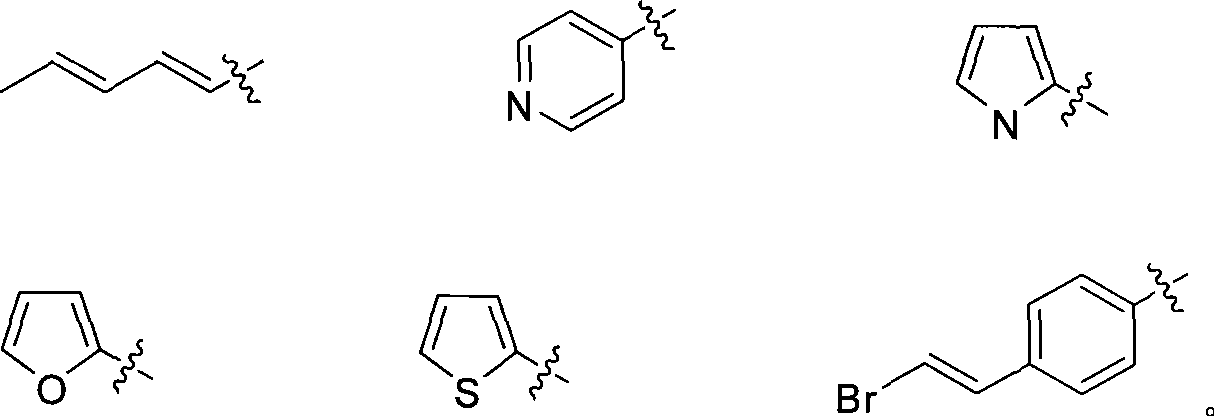
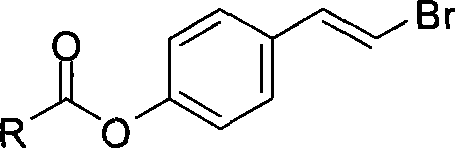
IDO restrainer containing (E)-4-(beta-bromo vinyl)benzoyloxy structure
The invention belongs to the technical field of new drug synthesis, and in particular relates to an IDO inhibitor containing (E)-4-(beta-bromovinyl)phenoxy acyl structure, as well as a preparation method thereof. The preparation method comprises the following steps: solvent benzene, (E)-4-(beta-bromovinyl) phenol, carboxylic acid and p-dimethylaminopyridine are added to a flask respectively and magnetically stirred for 5 to 20 minutes at room temperature; then N, N'-dicyclohexylcarbodiimide is added and reacts for 2 to 24 hours at room temperature; after the reaction is completed, the solvent benzene is steamed off after decompression; residue is subjected to column chromatography separation and purification by taking ethyl acetate / petroleum ether as leacheate, and then a needed product can be obtained, wherein the molar ratio of the solvent benzene to the (E)-4-(beta-bromovinyl) phenol is 50-200:1; the molar ratio of the carboxylic acid to the (E)-4-(beta-bromovinyl) phenol is 1-1.5:1; the molar ratio of the p-dimethylaminopyridine to the (E)-4-(beta-bromovinyl) phenol is 0.1-1.5:1; and the molar ratio of the N, N'-dicyclohexylcarbodiimide to the (E)-4-(beta-bromovinyl) phenol is 1-1.2:1. The method takes the (E)-4-(beta-bromovinyl) phenol as a synthesis building block and obtains a series of the novel IDO inhibitors containing the (E)-4-(beta-bromovinyl)phenoxy acyl structure through esterification reaction, and the IDO inhibitors can be used for treating the diseases with the pathological features of IDO mediated tryptophan metabolic pathway.
Owner:SHANGHAI TIANCI BIOLOGICAL VALLEY BIOLOGICAL ENG
Amino-pyridine-containing spleen tyrosine kinase (SYK) inhibitors
Owner:QUALCOMM INC +1
Solid forms of a selective cdk4/6 inhibitor
This invention relates to the crystalline free base of acetyl-8-cyclopentyl-5-methyl-2-(5-piperazin-1-yl-pyridin-2-ylamino)-8H-pyrido[2,3-d]pyrimidin-7-one, formula (1) having improved properties, to pharmaceutical compositions and dosage forms comprising the free base, and to methods for making and using such compounds, compositions and dosage forms in the treatment of cell proliferative diseases, such as cancer.
Owner:PFIZER INC
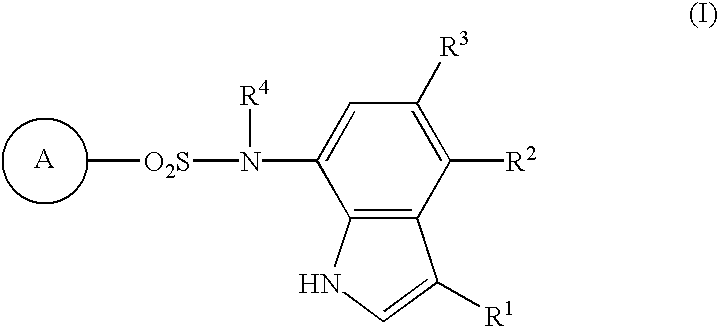
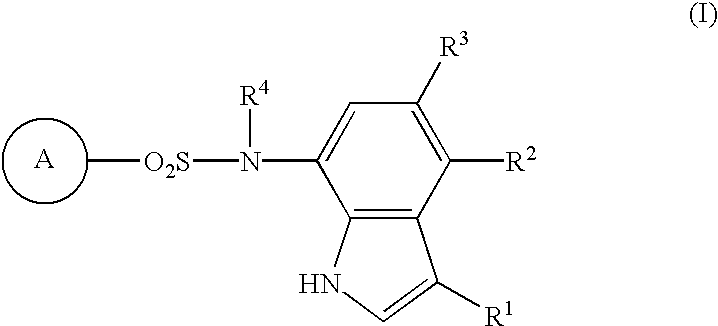
Sulfonamide-containing indole compounds
The present invention creates a novel antiangiogenic agent and provides an antitumor agent which shows high safety as compared with conventional antitumor agents, has a sure effect and is able to be administered for a long period. That is, it provides an indole compound represented by the following formula (I), its pharmacologically acceptable salt or hydrates thereof. 1 In the formula, R.sup.1 represents hydrogen atom, a halogen atom or cyano group; R.sup.2 and R.sup.3 are the same as or different from and each represents hydrogen atom, a C.sub.1-C.sub.4 lower alkyl group or a halogen atom; R.sup.4 represents hydrogen atom or a C.sub.1-C.sub.4 lower alkyl group; and the ring A represents cyanophenyl group, aminosulfonylphenyl group, aminopyridyl group, aminopyrimidyl group, a halopyridyl group or cyanothiophenyl group, provided that the case where all of R.sup.1, R.sup.2 and R.sup.3 are hydrogen atoms, where both R.sup.2 and R.sup.3 are hydrogen atoms or where the ring A is aminosulfonyl group and both R.sup.1 and R.sup.2 are halogen atoms is excluded. Further, when the ring A is cyanophenyl group, 2-amino-5-pyridyl group or a 2-halo-5-pyridyl group and R.sup.1 is cyano group or a halogen atom, at least one of R.sup.2 and R.sup.3 should not be a hydrogen atom.
Owner:EISIA R&D MANAGEMENT CO LTD
Aminopyridines useful as inhibitors of protein kinases
InactiveUS20110021559A1Surprising selectivityIncreasing axonalBiocideNervous disorderDiseasePTK Inhibitors
The present invention relates to compounds useful as inhibitors of protein kinases. The invention also provides pharmaceutically acceptable compositions comprising those compounds and methods of using the compounds and compositions in the treatment of various disease, conditions, and disorders. The invention also provides processes for preparing compounds of the invention.
Owner:VERTEX PHARMA INC
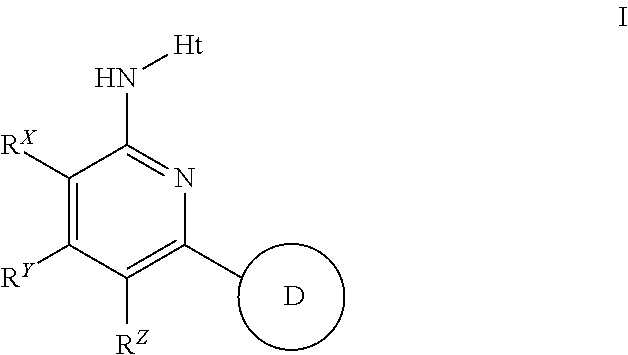
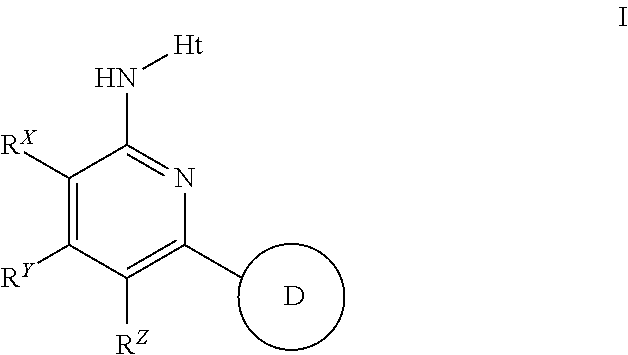
Novel 2-amino-pyridine and 2-amino-pyrimidine derivatives and medicinal use thereof
InactiveUS20170044133A1Superior in autotaxin inhibitory actionEffective prophylaxisOrganic active ingredientsNervous disorderAutotaxinDisease
Provided is a compound superior in an autotaxin inhibitory action and the like, effective as a prophylactic or therapeutic drug for diseases involving ATX. The present invention relates to a compound represented by the following formula (I):[wherein each symbol is as described in the DESCRIPTION], which has a superior autotaxin inhibitory action and is useful as a prophylactic or therapeutic drug for diseases involving ATX.
Owner:MITSUBISHI TANABE PHARMA CORP
A kind of synthetic method of ceftazidime
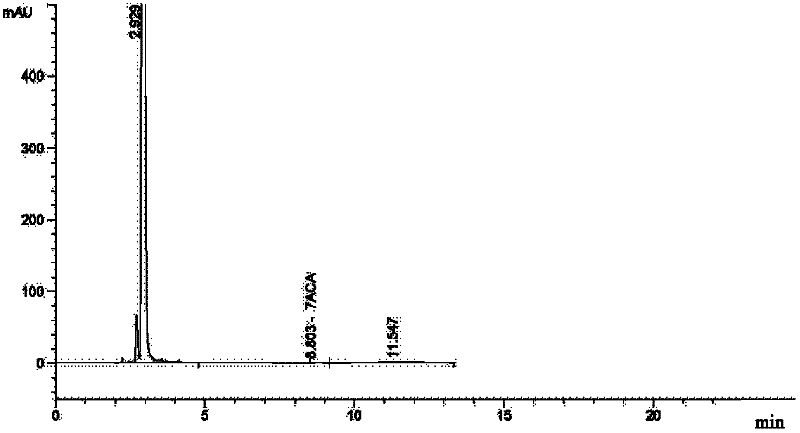
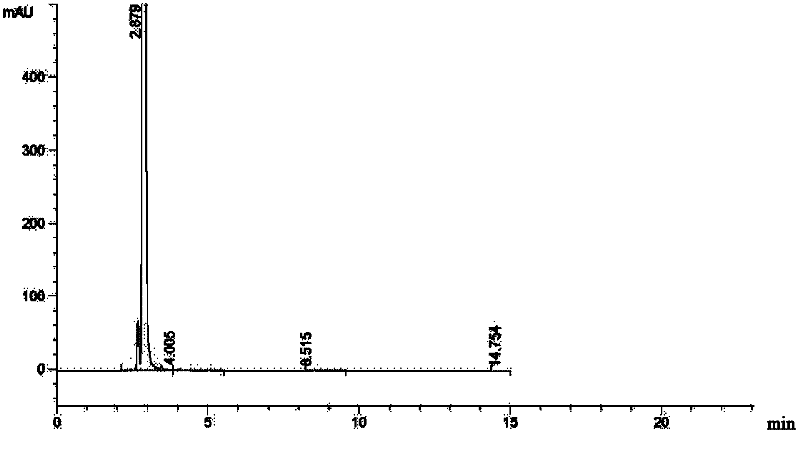
The invention relates to a method for preparing ceftazidime. The synthesis method comprises the following steps: by taking 7-aminocephalosporanic acid (7-ACA) as a starting raw material, introducing a pyridine ion to 3-position methylene of 7-ACA so as to obtain 7-amino-3-(1-picolyl)-cephem acid (7-APCA) hydrochloride; then introducing a side chain with a thiazole ring on 7-position amino of the 7-APCA hydrochloride through acylation reaction; and carrying out hydrolyzing reaction, refining reaction and the like so as to obtain ceftazidime. The synthesis method for preparing the ceftazidime inthe invention is simple to operate and high in yield, and is suitable for industrial production.
Owner:哈药集团股份有限公司 +1
Preparation method of aminopyridine modified resin adsorbing material
InactiveCN103588912ALarge adsorption capacityQuick responseOther chemical processesIndustrial waste waterIon
Owner:HEBEI UNIVERSITY OF SCIENCE AND TECHNOLOGY
Synergistic herbicidal compositions containing propyzamide and aminopyralid
An herbicidal composition containing (a) propyzamide and (b) aminopyralid provides synergistic control of selected weeds in oilseed rape and in broadleaf, grass and perennial crops.
Owner:CORTEVA AGRISCIENCE LLC
Enantioselective biotransformation for preparation of protein tyrosine kinase inhibitor intermediates
Owner:AGOURON PHARMA INC
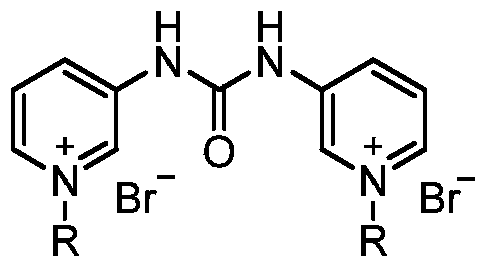
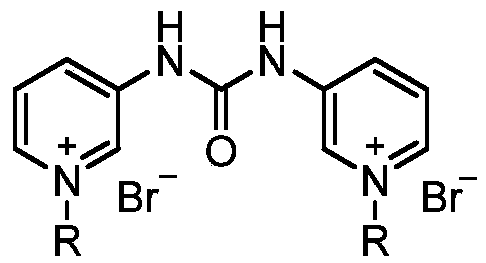
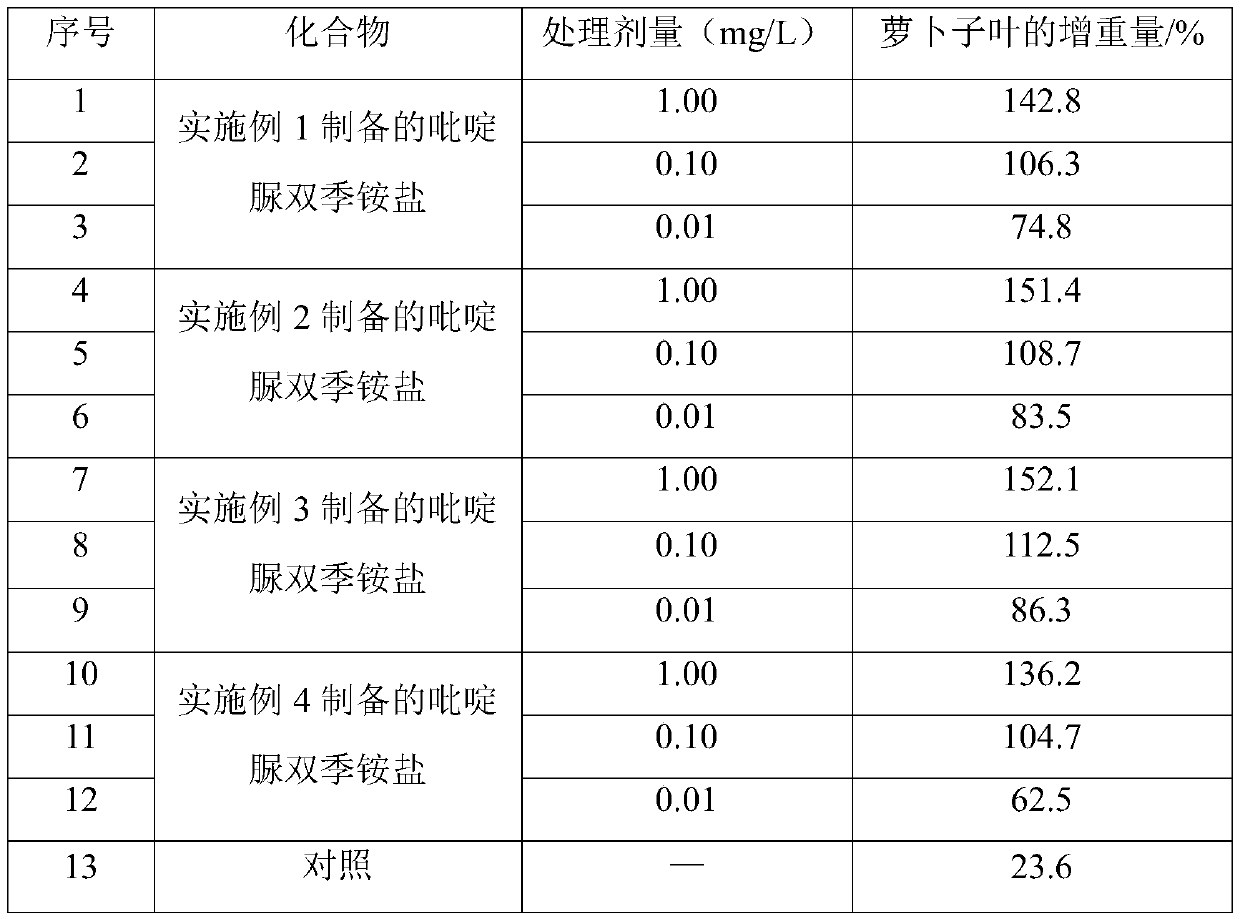
Pyridylurea biquaternary ammonium salt as well as preparation method and application thereof
ActiveCN105503711APromote divisionGood water solubilityPlant growth regulatorsBiocideSolubilitySolvent free
The invention relates to a pyridylurea biquaternary ammonium salt as well as a preparation method and application thereof. The preparation method of the pyridylurea biquaternary ammonium salt comprises the following steps: using 3-aminopyridine as a staring material, conducting reaction on 3-aminopyridine and triphosgene to obtain 1,3-bi(3-pyridyl)urea, and conducting reaction on 1,3-bi(3-pyridyl)urea and alkyl bromide under the solvent-free condition to prepare the pyridylurea biquaternary ammonium salt. According to the method, a solvent-free method is adopted to prepare the pyridylurea biquaternary ammonium salt, so that the environment can be protected, and the yield is relatively high. The provided pyridylurea biquaternary ammonium salt can be used as a plant growth regulator to promote plant cell division, and the problem that the traditional phenylurea plant growth regulator is poor in water solubility is solved.
Owner:SHANXI UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com
![2-(Pyridin-2-ylamino)-pyrido[2,3-d]pyrimidin-7-ones 2-(Pyridin-2-ylamino)-pyrido[2,3-d]pyrimidin-7-ones](https://images-eureka.patsnap.com/patent_img/211494ac-0ef3-4b13-a9b2-61f9ab91cee8/US06936612-20050830-C00001.png)
![2-(Pyridin-2-ylamino)-pyrido[2,3-d]pyrimidin-7-ones 2-(Pyridin-2-ylamino)-pyrido[2,3-d]pyrimidin-7-ones](https://images-eureka.patsnap.com/patent_img/211494ac-0ef3-4b13-a9b2-61f9ab91cee8/US06936612-20050830-C00002.png)
![2-(Pyridin-2-ylamino)-pyrido[2,3-d]pyrimidin-7-ones 2-(Pyridin-2-ylamino)-pyrido[2,3-d]pyrimidin-7-ones](https://images-eureka.patsnap.com/patent_img/211494ac-0ef3-4b13-a9b2-61f9ab91cee8/US06936612-20050830-C00003.png)