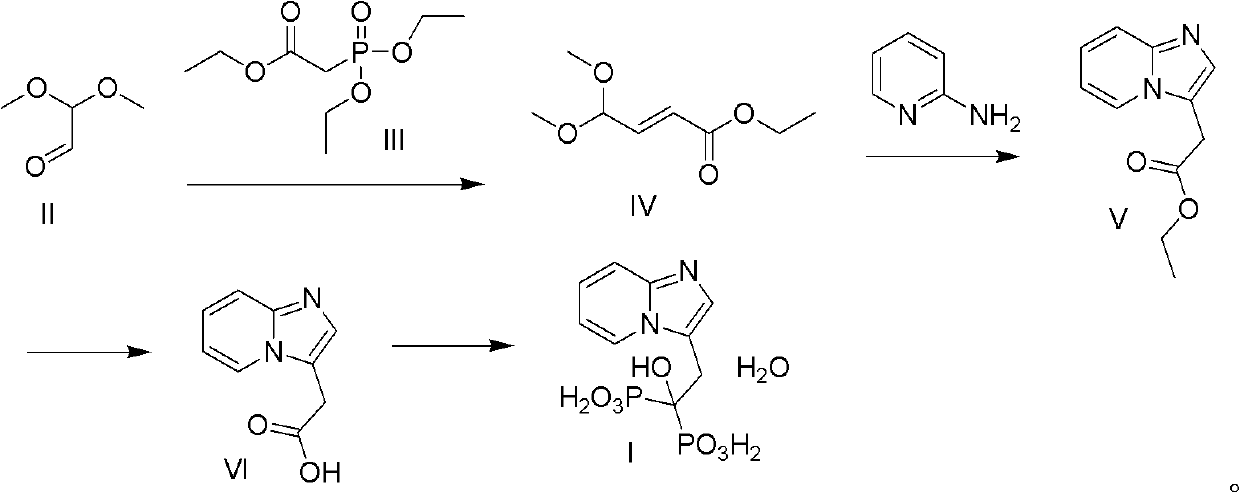
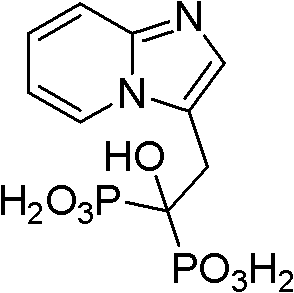
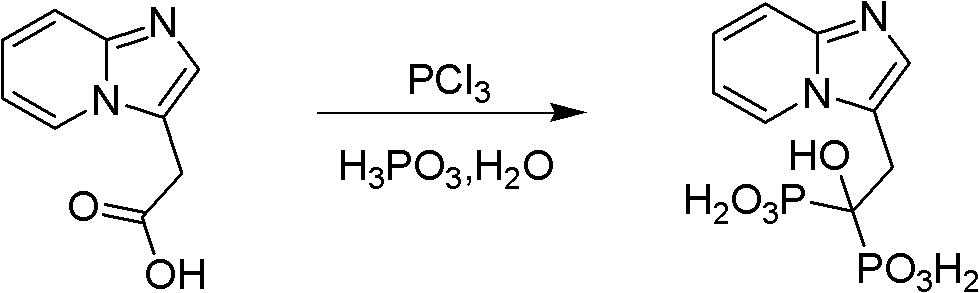
Method for preparing minodronate
A phosphorylation and compound technology, which is applied in the field of minophosphoric acid preparation, can solve the problems of being unsuitable for large-scale production in the pharmaceutical industry, high toxicity, and difficulty in realization, and achieves low cost, short reaction steps, and mild and controllable reaction conditions. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] 1. Preparation of (E)-4,4-dimethoxy-2-butenoic acid ethyl ester
[0029] Add 120g of 2,2-methoxyacetaldehyde and 4L of cyclohexane into a four-neck flask, add 900g of anhydrous potassium carbonate while stirring, and stir for 1h. Add 500 mL of triethyl phosphonoacetate dropwise and react for 6 h. After the reaction was completed, 1.5 L of water was added to the reaction liquid, and the liquids were separated. The organic phase was washed with saturated brine, dried over anhydrous sodium sulfate, and the solvent was evaporated under reduced pressure to obtain a colorless oily liquid. The oily liquid was distilled under reduced pressure to obtain a colorless Oil 150g, yield 71%.
[0030] 2, Preparation of 2-(imidazo[1,2-a]pyridin-3-yl) ethyl acetate
[0031] Under the protection of nitrogen, 200g of ethyl (E)-4,4-dimethoxy-2-butenoate, 1000mL of acetonitrile and 100mL of water were added to a three-necked flask, and then 10g of p-toluenesulfonic acid was added and stirr...
Embodiment 2
[0037] 1. Preparation of (E)-4,4-dimethoxy-2-butenoic acid ethyl ester
[0038] Add 180g of 2,2-dimethoxyacetaldehyde, 1300g of anhydrous potassium carbonate and 5800mL of cyclohexane into a round bottom flask, stir, add 670mL of triethyl phosphonoacetate dropwise, and react for 6h after the addition. After the reaction, add 2200mL, separate the layers, wash the organic layer with saturated brine, dry over anhydrous sodium sulfate, filter off the desiccant, concentrate the filtrate under reduced pressure to obtain a colorless oily liquid, distill the oily liquid under reduced pressure to obtain 210g of the product , yield 70%.
[0039] 2, Preparation of 2-(imidazo[1,2-a]pyridin-3-yl) ethyl acetate
[0040] Under nitrogen protection, add 196g (E)-4,4-dimethoxy-2-butenoic acid ethyl ester, 1000mL acetonitrile and 80mL water into the three-necked flask, stir, add 10g p-toluenesulfonic acid, then add 65g 2- Aminopyridine was heated and refluxed for 4h. After the reaction, filte...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com