Patents
Literature
219 results about "2-Aminopyridine" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
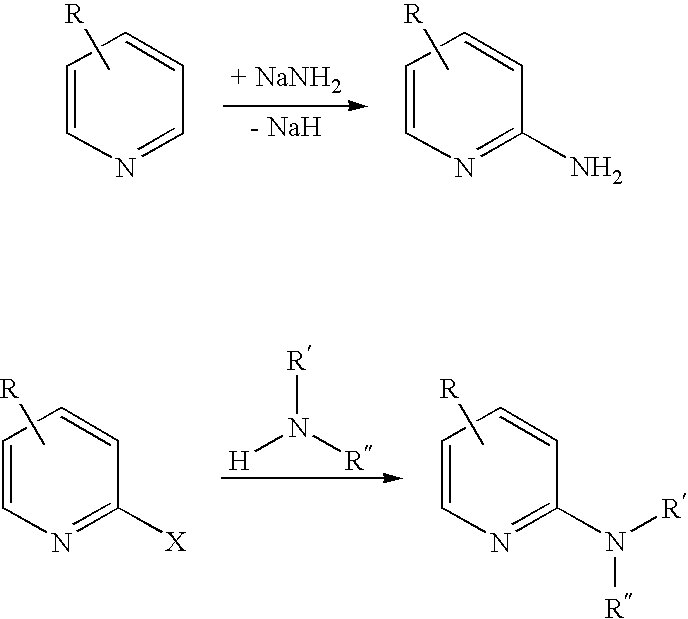
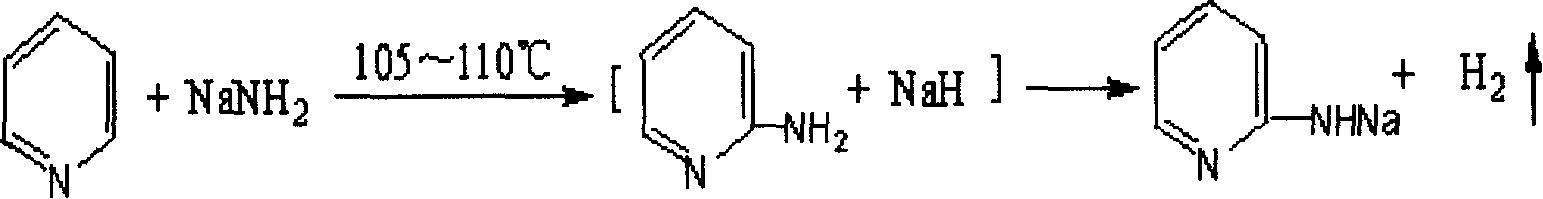
2-Aminopyridine is an organic compound with the formula H₂NC₅H₄N. It is one of three isomeric aminopyridines. It is a colourless solid that is used in the production of the drugs piroxicam, sulfapyridine, tenoxicam, and tripelennamine. It is produced by the reaction of sodium amide with pyridine, the Chichibabin reaction.
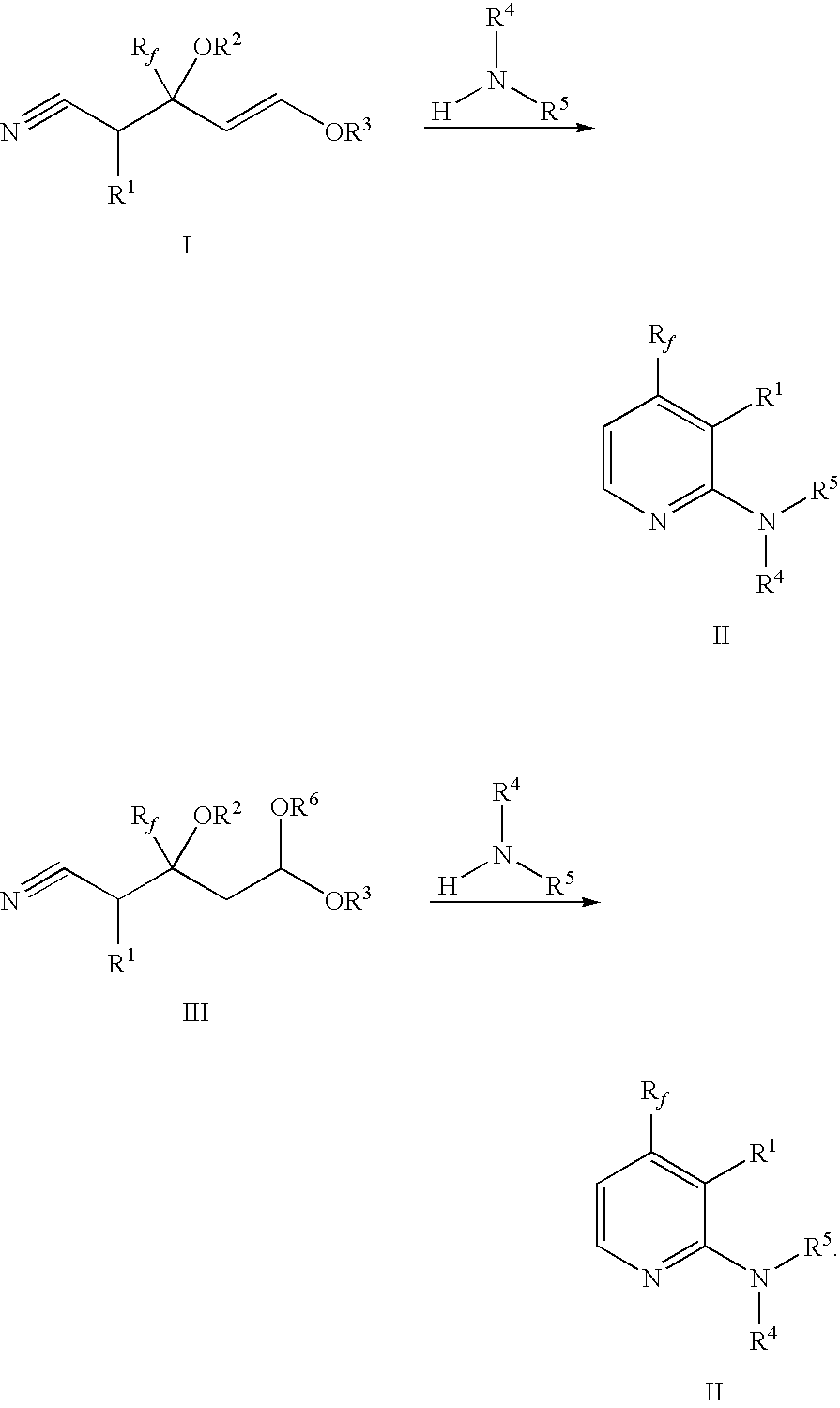
2-(Pyridin-2-ylamino)-pyrido[2,3-d]pyrimidin-7-ones
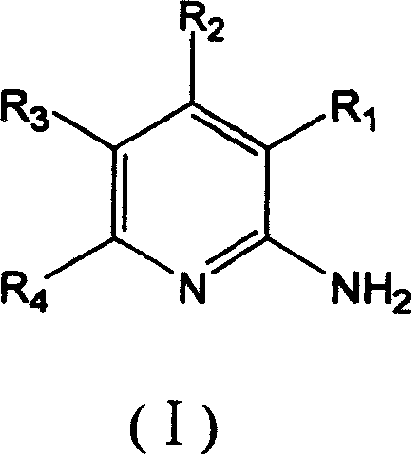
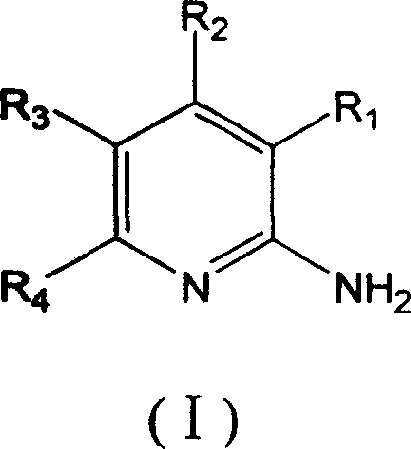
The present invention provides substituted 2-aminopyridines useful in treating cell proliferative disorders. The novel compounds of the present invention are potent inhibitors of cyclin-dependent kinases 4 (cdk4)
Owner:WARNER LAMBERT CO LLC
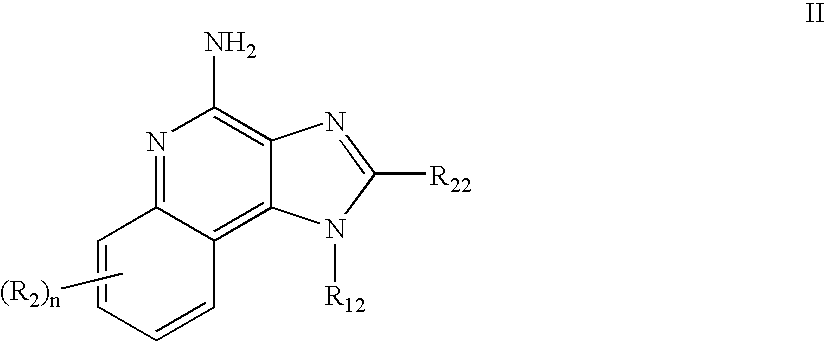
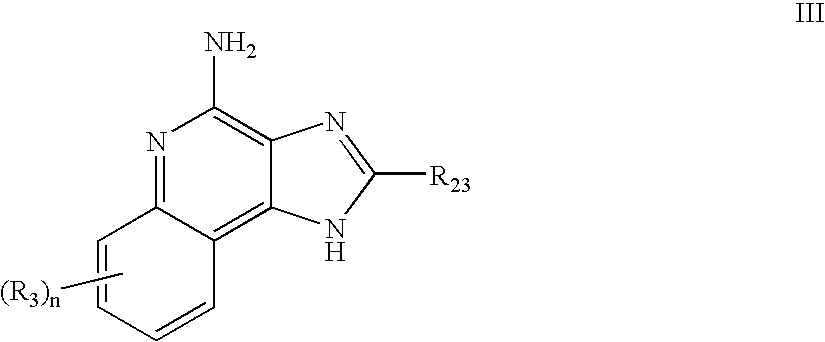
Immune response modifier formulations and methods
Pharmaceutical formulations including an immune response modifier (IRM) compound having a 2-aminopyridine moiety fused to a five-membered nitrogen-containing heterocyclic ring; a preservative system including a sorbic acid preservative selected from the group consisting of sorbic acid, esters thereof, salts thereof, and combinations thereof; an antioxidant; and an optional chelating agent.
Owner:MEDICIS PHARMA CORP
2-aminopyridine kinase inhibitors
InactiveUS20090197862A1Useful in treatmentReduce usageBiocideOrganic chemistryKinase inhibitionEnzyme inhibitor
2-Aminopyridine compounds having the structure of Formula I, and pharmaceutically acceptable salts of these compounds. Compounds of Formula I inhibit the activity of tyrosine kinase enzymes in animals, including humans, and are useful in the treatment and / or prevention of various diseases and conditions. In particular, compounds disclosed herein are inhibitors of kinases, in particular, but not limited to, KDR, Tie-2, Flt3, FGFR3, Ab1, Aurora A, c-Src, IGF-1R, ALK, c-MET, RON, PAK1, PAK2, and TAK1, and can be used in the treatment of proliferative diseases, such as, but not limited to, cancer. The present invention is also directed to a pharmaceutical composition comprising a therapeutically effective amount of a compound of Formula I, or a pharmaceutically acceptable salt thereof, and a pharmaceutically acceptable carrier. The present invention is further directed to a method of treating a patient having a condition which is mediated by protein kinase activity by administering to the patient a therapeutically effective amount of the above-mentioned pharmaceutical composition.
Owner:OSI PHARMA LLC
2-Aminopyridine compounds and use thereof as drugs
The present invention provides 2-aminopyridine compound having an excellent adenosine receptor (A1, A2a, A2b receptors) antagonism, which is represented by the following formula: (wherein, R<1 >represents cyano group, carboxyl group or an optionally substituted carbamoyl group; R<2 >represents hydrogen atom, hydroxyl group, an optionally substituted C1-6 alkoxy group, an optionally substituted C6-14 aromatic hydrocarbon cyclic group or an optionally substituted 5- to 14-membered aromatic heterocyclic group; and R<3 >and R<4 >are the same as or different from each other and each represents a C6-14 aromatic hydrocarbon cyclic group, a 5- to 14-membered non-aromatic heterocyclic group or a 5- to 14-membered aromatic heterocyclic group which may be substituted, respectively) or a salt thereof.
Owner:EISIA R&D MANAGEMENT CO LTD
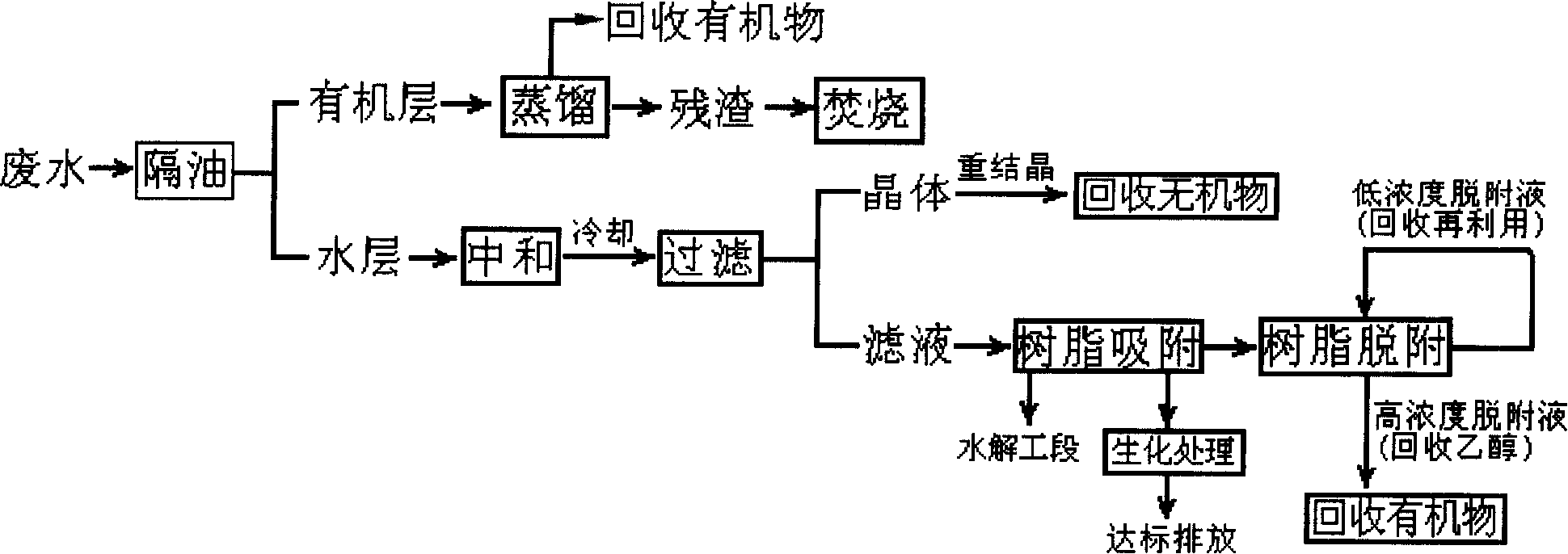
Method for treating and reclaiming waste water of 2-aminopyridine production
InactiveCN101235014AImprove governance effectOrganic chemistryNature of treatment waterRecovery methodDesorption
The invention provides a treatment and recovery method of 2-aminopyridine production waste water, comprising separating organic layer from waste water via an oil separation pool, depressurizing, distilling and recovering 2-aminopyridine, adjusting pH of aqueous layer via a diluted acid until neutral, cooling, crystallizing, depressurizing, extracting and filtering to obtain crude salt, purifying and recovering inorganic salt via recrystallization, using adsorption column stuffed with high-crosslinked adsorption resin to absorb and remove organics, collecting effluent to be used for hydrolysis or be discharged via biochemical treatment, eluting the adsorption resin via sodium-hydroxide-alcohol solution to be regenerated via methanol to be repeatedly used, neutralizing and combining the desorption solution and organic layer, distilling to recover 2-aminopyridine. The treatment and recovery method of 2-aminopyridine production waste water has the advantages of improved waste water treatment effect and recovered useful material in waste water, thereby reducing pollution, saving cost and combining waste water treatment and resource recovery.
Owner:YANCHENG TEACHERS UNIV
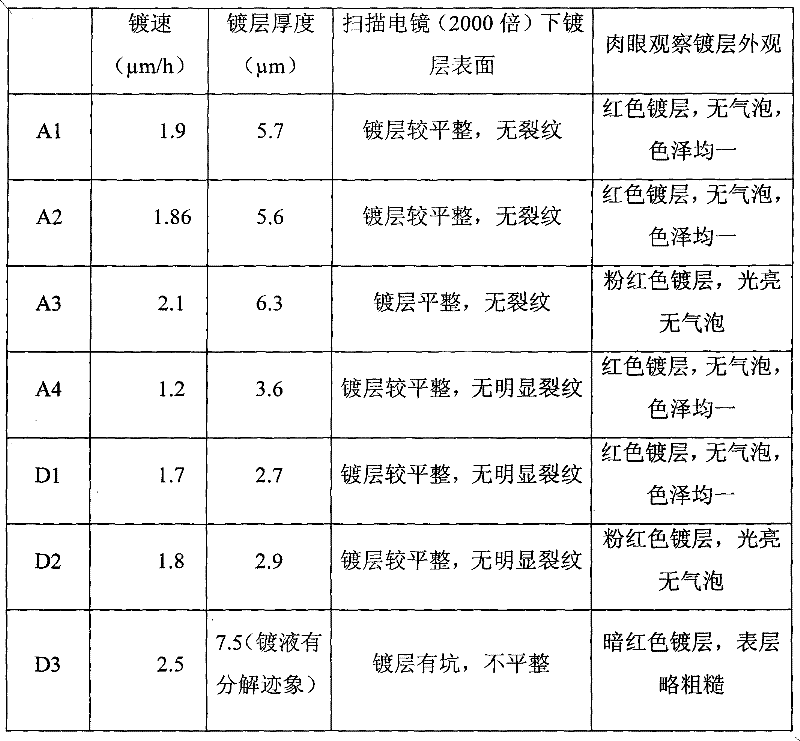
Chemical copper-plating solution and chemical copper-plating method
ActiveCN102534583AIncrease the maximum thicknessLiquid/solution decomposition chemical coatingNickel saltCopper plating
The invention provides chemical copper-plating solution and a chemical copper-plating method. The chemical copper-plating solution comprises copper salt, nickel salt, sodium monophosphate, a complexing agent, a stabilizing agent and water solution of a potential of hydrogen (pH) conditioning agent. The stabilizing agent contains 2-aminopyridine and 4 cyanopyridine. The complexing agent comprises triethanolamine and citrate, the content ratio of the triethanolamine to the citrate is 1:1-5:1, and the pH value of the chemical copper-plating solution is 8-11. By adopting the chemical copper-plating solution with the sodium monophosphate serving as a reducing agent, the thickness of a plating layer can reach 3-7 micrometers, so that the largest thickness of a traditional technology is greatly improved.
Owner:BYD CO LTD
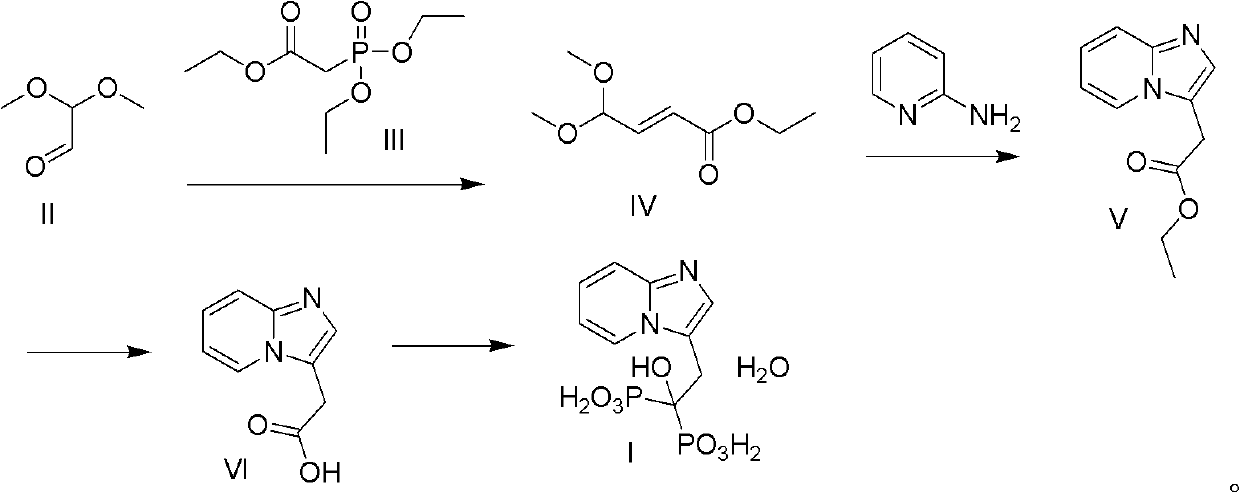
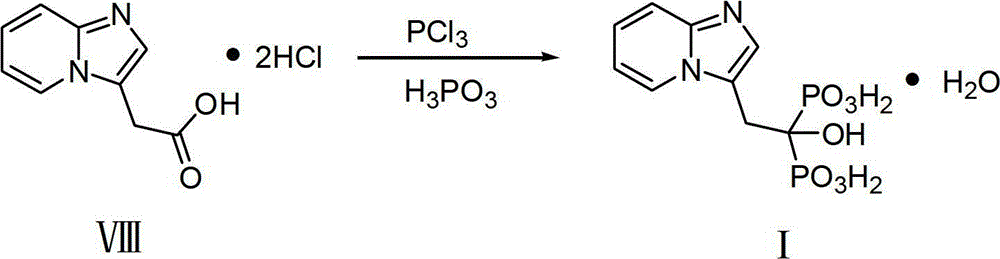
Method for preparing minodronate
ActiveCN102020676AThe reaction conditions are mild and controllableShort reaction stepsGroup 5/15 element organic compoundsBromineSodium cyanide
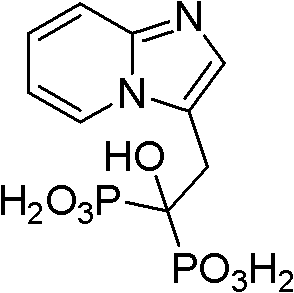
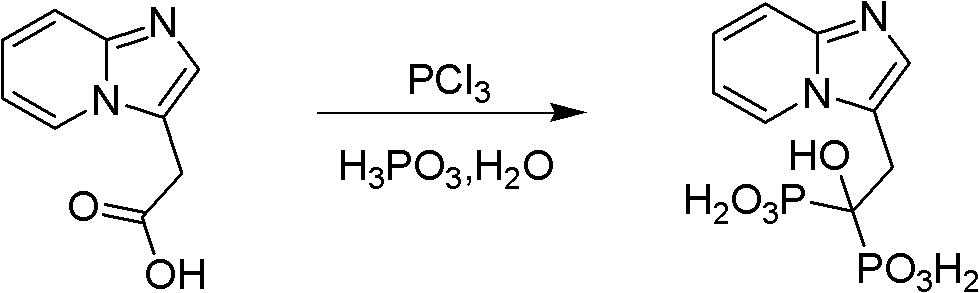
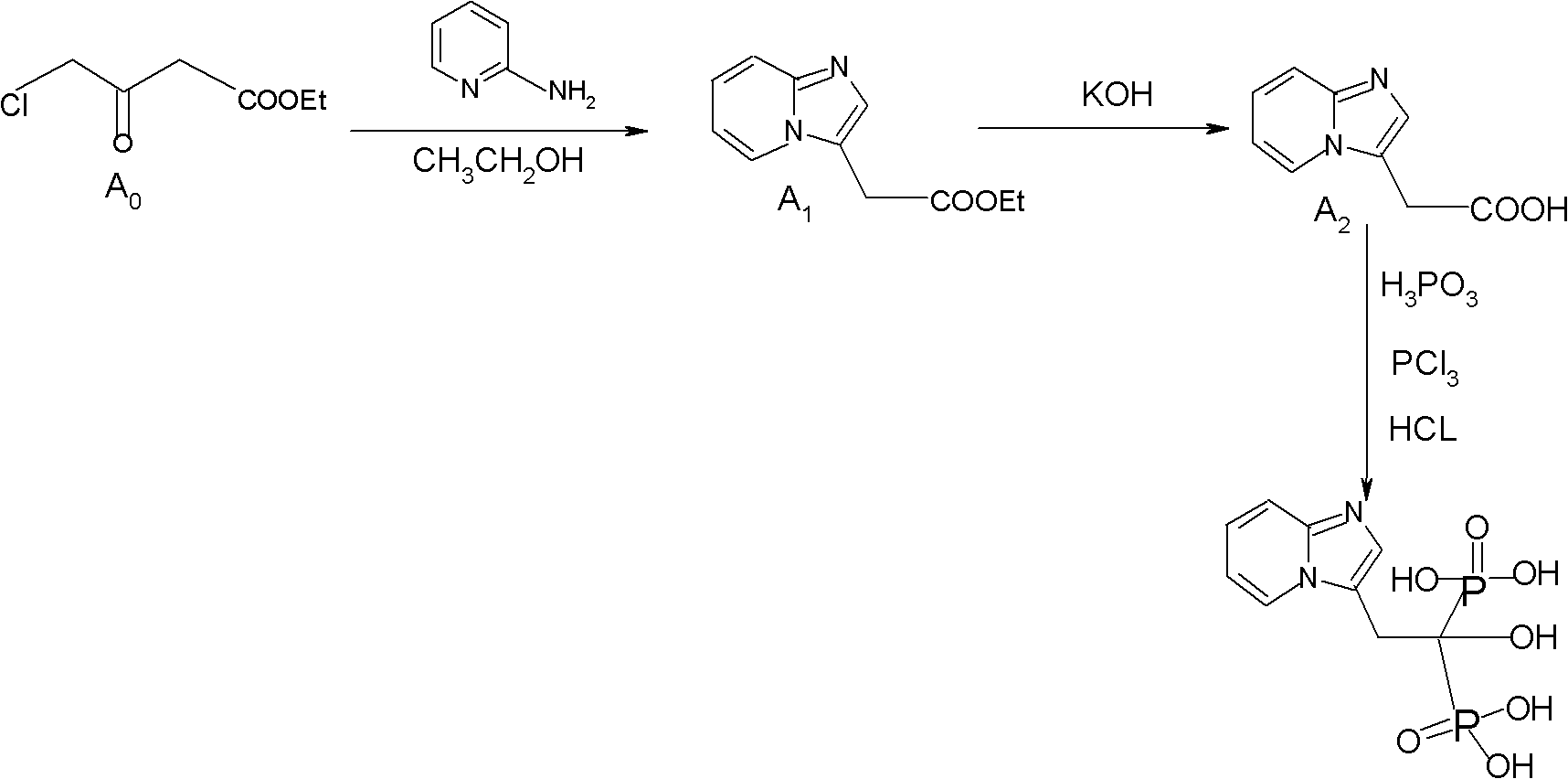
The invention discloses a method for preparing minodronate, which comprises the following steps of: condensing a compound II and a compound III to form a compound IV, closing rings of the compound IV and 2-aminopyridine to obtain a compound V, hydrolyzing the compound V into a compound VI, and finally performing phosphonation to form a compound I. The method avoids using virulent chemical reagents such as sodium cyanide or bromine and the like, has mild and controllable reaction condition, short reaction step, high yield and low cost, and is suitable for industrialized production.
Owner:JIANGSU LEEWAY BIOLOGICAL TECH
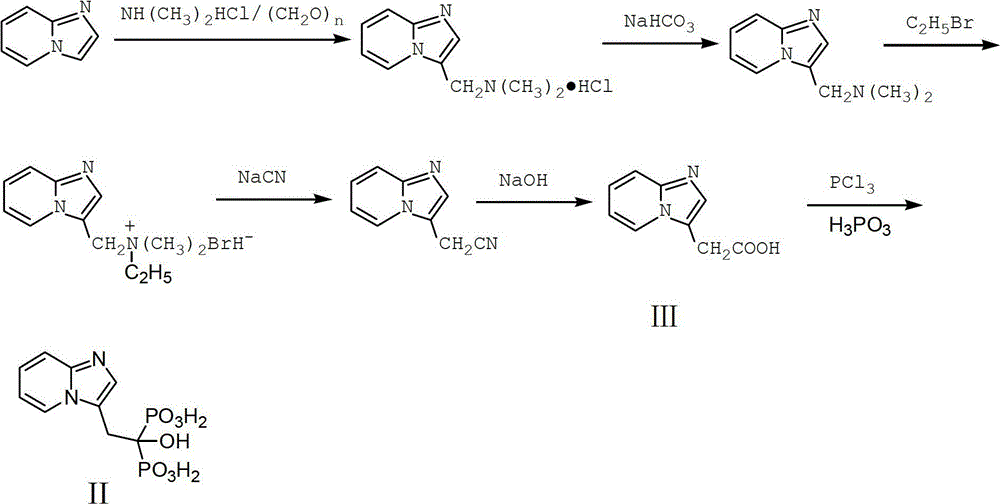
Novel method for preparing important intermediate of minodronate
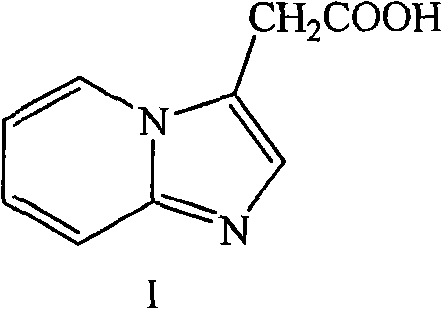
The invention relates to a method for preparing 2-(imidazo[1,2-a]pyridine-2-pyridinyl) acetic acid of a compound in a formula (I). In the method, trans-4-oxo-2-crotonate and 2-aminopyridine in a compound of a formula (II) perform a condensation reaction, and are hydrolyzed to form the compound of the formula (I). The compound is an important intermediate for preparing the minodronate.
Owner:北京京卫信康医药科技发展有限公司
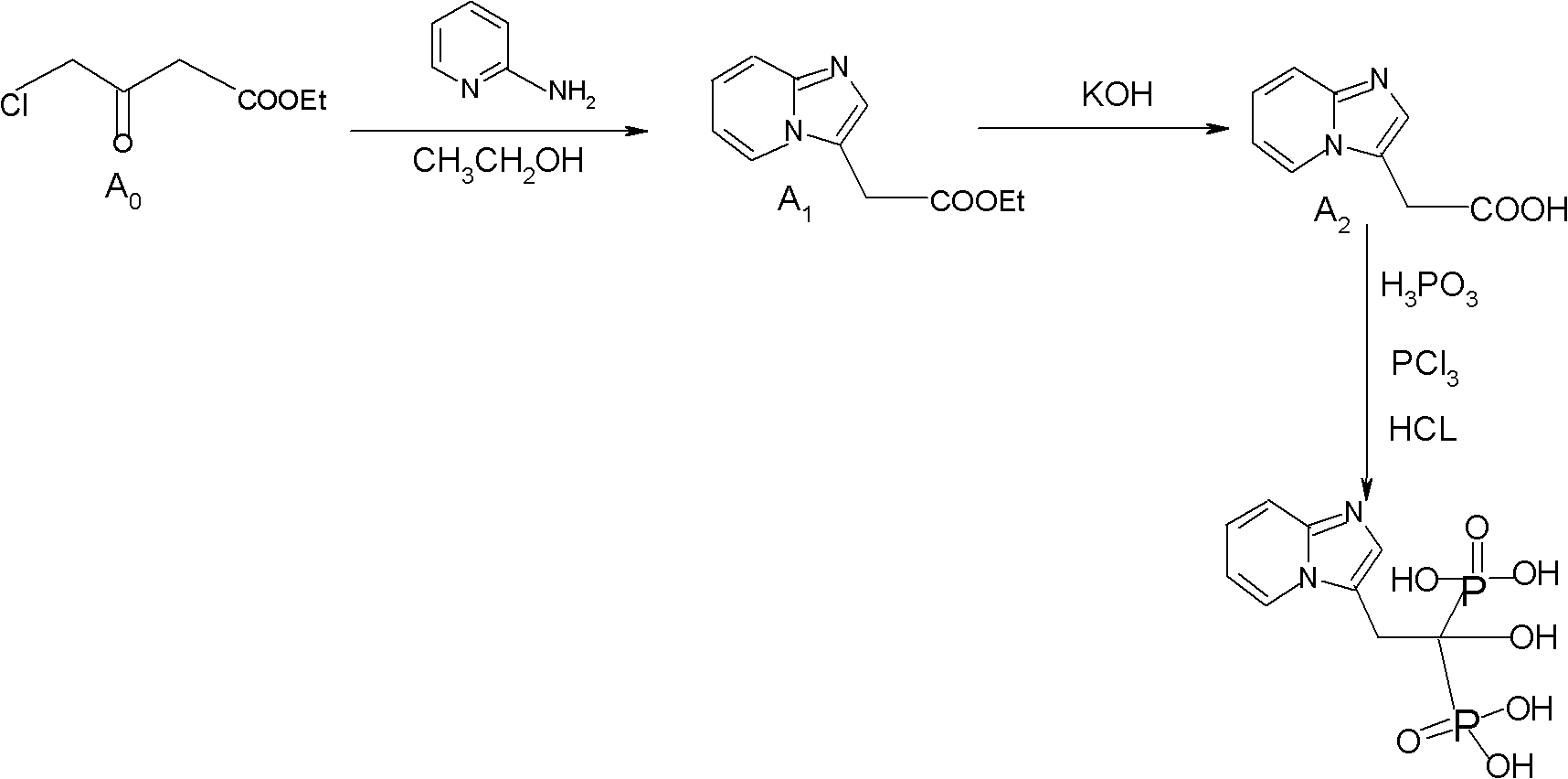
Synthesis method of minodronate midbody and synthesis of minodronate
ActiveCN102153585AAvoid pollutionImprove securityGroup 5/15 element organic compoundsSynthesis methodsFiltration
The invention relates to the field of pharmaceutical chemistry, in particular to a synthesis method of a minodronate midbody and synthesis of minodronate. The preparation method of minodronate includes the following steps: using organic solvent to dissolve 2-aminopyridine, adding 4-acetyl chloride ethyl acetoacetate for reaction, monitoring the reaction solution by TLC(Thin-Layer Chromatography) until spots of 4-acetyl chloride ethyl acetoacetate disappear, concentrating to a dry state, dissolving concentrate in water, washing a water layer to remove impurities, extracting the water layer with the organic solvent, washing extract liquor, separating out an organic layer, conducting filtration, and concentrating the concentrate to be in a dry state, thereby obtaining A1.
Owner:福建太平洋制药有限公司
Substituted 2-aminopyridine inhibitor for protein kinase
InactiveCN103965161AOrganic active ingredientsGroup 5/15 element organic compoundsPyridineIsrapafant
The invention discloses a substituted 2-aminopyridine inhibitor for protein kinase, and concretely relates to 2-aminopyridine derivatives with protein kinase inhibition activity, a preparation method thereof, and pharmaceutical compositions thereof, and the invention also discloses application of the compounds and the pharmaceutical compositions thereof to treat diseases related to protein kinase.
Owner:CHIA TAI TIANQING PHARMA GRP CO LTD +2
Enantioselective biotransformation for preparation of protein tyrosine kinase inhibitor intermediates
Owner:AGOURON PHARMA INC
Method for preparing pyridinoquinazolinone compound through catalysis of copper compound
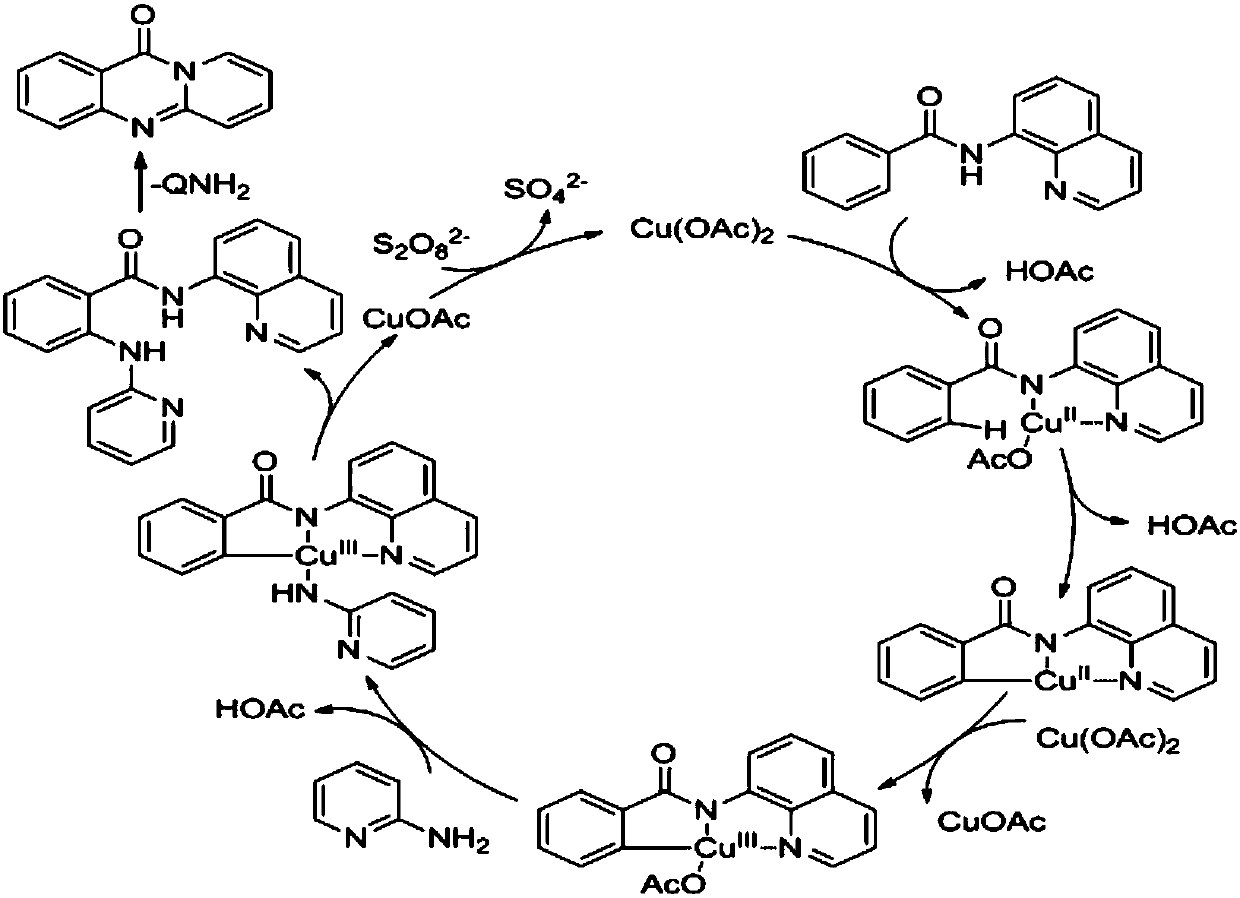
The invention belongs to the fields of organic synthesis and metal catalysis, and discloses a method for preparing a pyridinoquinazolinone compound through the catalysis of a copper compound. The method comprises the following steps: adding a benzamide derivative, a 2-aminopyridine derivative, a catalyst, an oxidizing agent and a solvent into a closed pipe in an air environment; then performing aheating reaction; and after the reaction is completed, purifying the obtained reaction solution to obtain the required pyridinoquinazolinone compound. According to the method, a C-H activation cascadereaction is performed on the benzamide derivative and the 2-aminopyridine derivative under the catalytic action of the copper compound in the presence of the oxidizing agent in the air environment and the closed environment, thereby generating the pyridinoquinazolinone; the method can be performed in the air environment; and the used raw materials, catalyst and oxidizing agent are simple and accessible, the reaction is simple to operate, and the yield is high, thereby being beneficial to industrial production.
Owner:GUANGZHOU UNIVERSITY
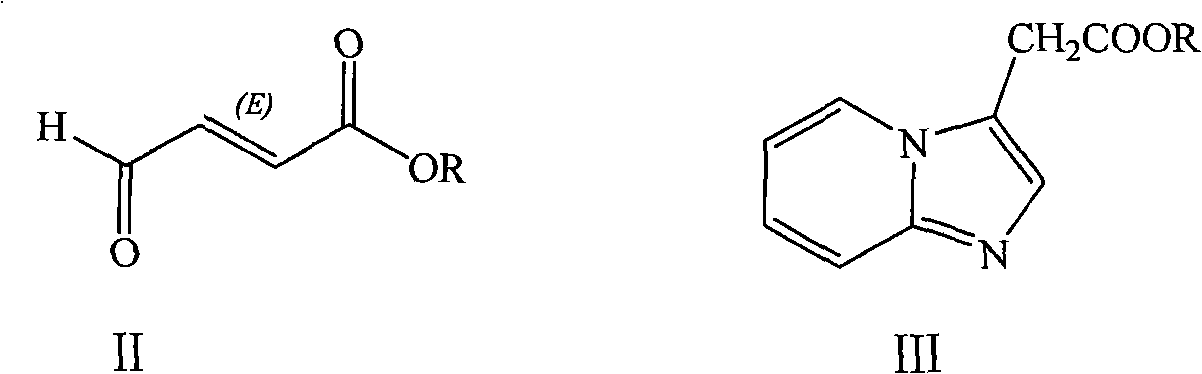
Method for preparing 2-[imidazo[1,2-a]pyridine-3-yl]acetic acid
The invention provides a method for preparing 2-[imidazo[1,2-a]pyridine-3-yl]acetic acid, which comprises the following steps of: reacting 4-chloro-acetoacetic acid ester and 2-aminopyridine in an organic solvent in the presence of an acid binding agent to obtain 2-[imidazo[1,2-a]pyridine-3-yl]acetic acid ester; and hydrolyzing the 2-[imidazo[1,2-a]pyridine-3-yl]acetic acid under the acid or alkaline condition to obtain the 2-[imidazo[1,2-a]pyridine-3-yl]acetic acid. In the method, raw materials have high stability and low price and are readily available; deadly toxic cyanides or high corrosion and unstable bromides are not used; the reaction step is short; the operation is simple; the method is safe and environment-friendly; special equipment is not needed; and the method is suitable for industrial production.
Owner:TIANJIN INSTITUTE OF PHARMA RESEARCH
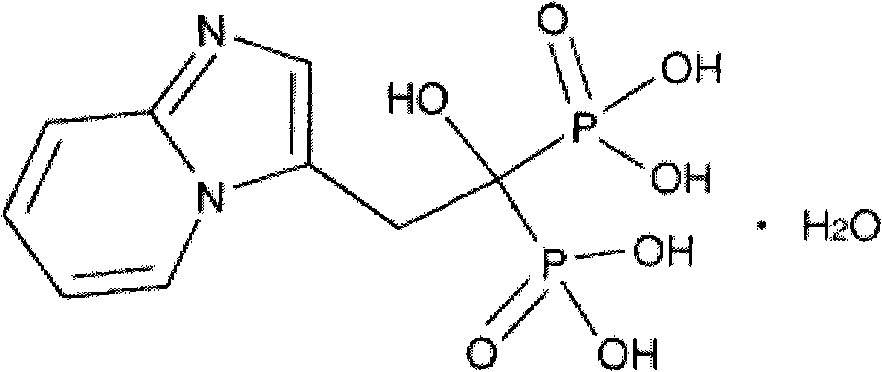
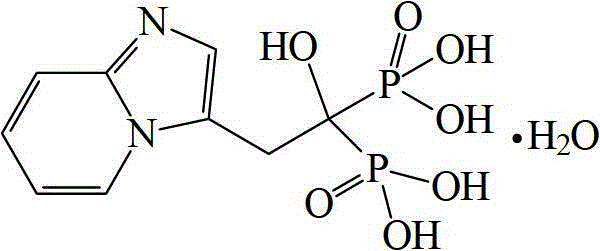
Preparation method of Minodronic acid hydrate
ActiveCN102875602ASufficient sourceLow costGroup 5/15 element organic compoundsMinodronic acid hydrateAfter treatment
The invention discloses a preparation method of Minodronic acid hydrate, which comprises the following steps: carrying out ketonic group protection on a compound (VII) to obtain a compound (VI), carrying out nucleophilic substitution reaction on the compound (VI) and 2-aminopyridine to obtain a compound (V), carrying out ketonic group deprotection on the compound (V), and carrying out cyclization reaction to obtain a compound (IV); and hydrolyzing the compound (IV) to obtain a compound (III), carrying out diphosphorylation on the compound (III) to obtain a compound (II), and recrystallizing the compound (II) to obtain the Minodronic acid hydrate (I). The synthetic route disclosed by the invention has the advantages of sufficient initial raw material sources, low cost, environment-friendly reagents used in the reaction process, safe industrial production, mild reaction conditions, fewer side reaction, simple after-treatment and no need of special or complex reaction equipment, is convenient to operate, and can easily implement industrial production.
Owner:JIANGSU SHENLONG PHARMA
Efficient heavy metal sludge treating agent
InactiveCN106587550AHigh degree of recoveryLow costWater contaminantsWaste based fuelBenzoic acidSodium Bentonite
The invention discloses an efficient heavy metal sludge treating agent. The efficient heavy metal sludge treating agent is prepared from, by weight, 5-12 parts of glutamine, 25-35 parts of sodium bentonite, 5-10 parts of methylparaben, 10-20 parts of activated carbon, 5-8 parts of polydiallyldimethylammonium chloride, 2-8 parts of p-chloromethyl benzoic acid, 2-6 parts of 2-aminopyridine-4-methanol, 5-12 parts of biogas residues, 5-10 parts of waste culture media for edible fungi, 2-4 parts of degrading bacteria, 10-20 parts of bentonite and 0.2-0.4 part of ferrous sulfate. Physical and chemical indicators of soil heavily contaminated by heavy metals can reach the GB15618-2008 standard; the treatment technology is simple, the dosage is small, the treatment effect is good, the performance is stable, the contaminated soil recovery degree is high, the cost of the treating agent can be effectively lowered, and the treating agent has good economic benefits and extensive social benefits.
Owner:ZHENGZHOU BEIDOU COMM TECH
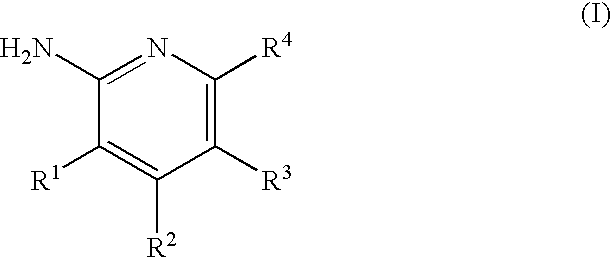
2-(Pyridin-2-ylamino)-pyrido [2,3-D]pyrimidin-7-ones
InactiveUS20050137214A1Ease of detectabilityEasy to prepareBiocideOrganic active ingredientsBiochemistryCyclin
The present invention provides substituted 2-aminopyridines useful in treating cell proliferative disorders. The novel compounds of the present invention are potent inhibitors of cyclin-dependent kinases 4 (cdk4).
Owner:WARNER-LAMBERT CO
Filgotinib synthetic method
ActiveCN104987333AWon't happenLess impuritiesOrganic chemistryChemical synthesisTert-Butyloxycarbonyl protecting group
The invention discloses a filgotinib synthetic method and belongs to the technical field of chemical synthesis of medicines. 6-chloro-2-aminopyridine and di-tert-butyl dicarbonate ester are subjected to condensation reaction to obtain 6-chloro-2-tert-butyloxycarbonyl aminopyridine; hydrolysis reaction is performed; the obtained 6-chloro-2-tert-butyloxycarbonyl aminopyridine and trifluorinated methyl sulfonic anhydride are subjected to trifluoromethanesulfonic acid esterification reaction; the obtained 2-tert-butyloxycarbonylamino-6-pyridyltrifluoromethanesulfonate and [(1,1-dioxo-4-thiomorpholinyl)methyl]benzo-4-boronic acid pinacol ester are subjected to condensation reaction to obtain a tert-butyl ester derivative; the tert-butyl ester derivative is treated by trifluoroacetic acid and subjected to de-protection; the obtained intermediate and ethoxycarbonyl isothiocyanate are subjected to isothiocyanate reaction; the obtained intermediate and hydroxylamine hydrochloride are subjected to ring closing reaction; the obtained intermediate and cyclopropane carbonyl chloride are subjected to amidation reaction to obtain the finished product. Operation is simplified; reagents are available; the concept of environment friendliness and environment protection is embodied.
Owner:SUZHOU FUSHILAI PHARMA CO LTD
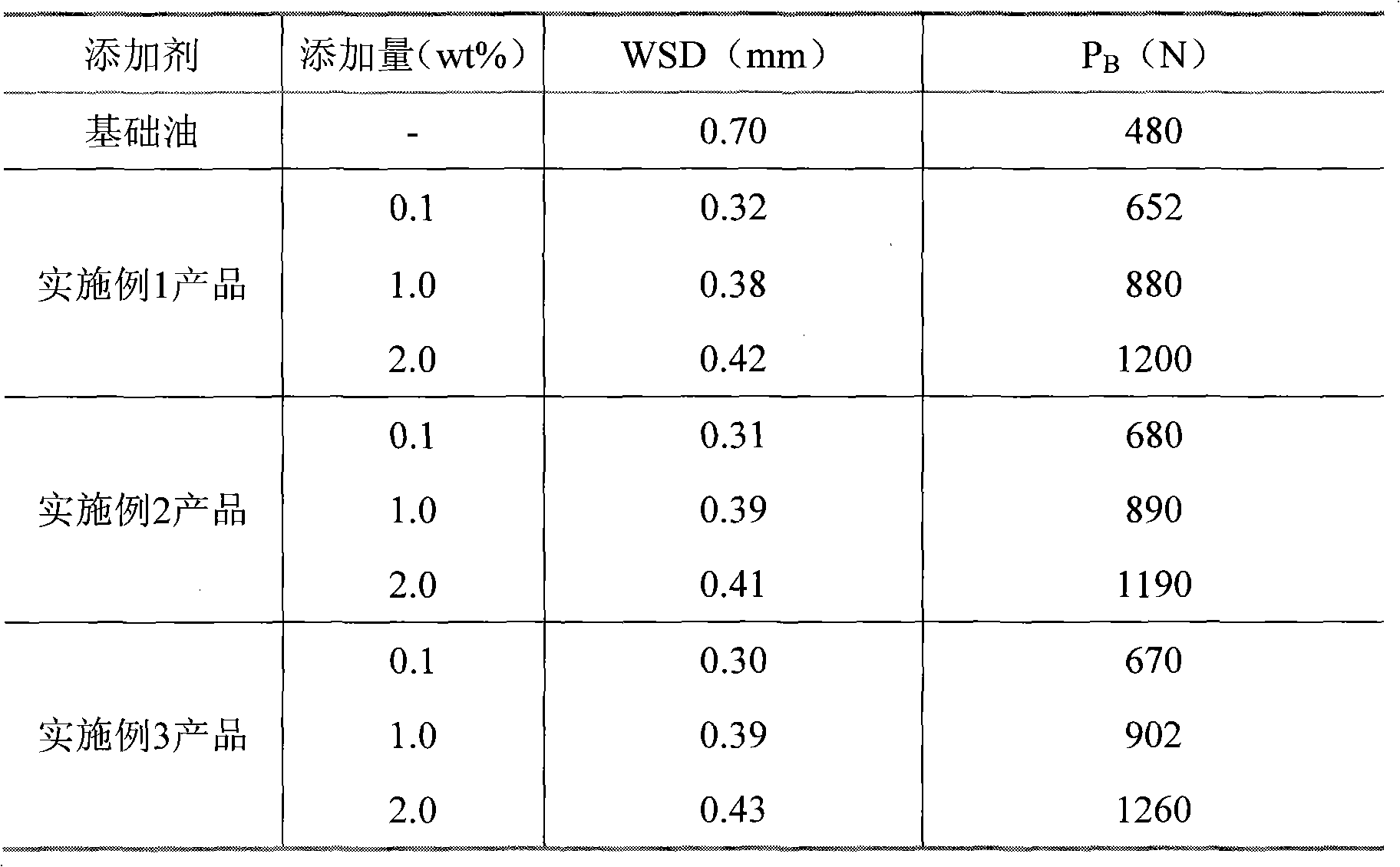
Additive for wear resistance of lubricating oil
ActiveCN101812354AExcellent extreme pressure anti-wear and anti-friction propertiesNot corrosiveAdditivesParaffin waxPolybutene
The invention discloses an additive for wear resistance of lubricating oil, which comprises the following components in part by weight: 15 to 35 parts of antiwear agent, 6 to 29 parts of detergent dispersant and 12 to 26 parts of antioxidant preservative, wherein the antiwear agent consists of boric acid tributylester, phosphonic acid dibutyl ester, copper isooctoxyborate and cupric cyclohexoxylborate; the detergent dispersant consists of polybutene amine, polyisobutene carbamic acid ester and 2-aminopyridine; and the antioxidant preservative is boronized butanimide. The additive has wide use range, can be used in paraffin, naphthene and intermediate base oil, and also can be used in synthetic and semi-synthetic base oil. The additive has the advantages of effectively improving the wear resistance of the lubricating oil, strengthening the bearing capacity of the lubricating oil, making the lubricating oil have long service life, and having small dosage in the lubricating oil and simple and convenient preparation process.
Owner:山东星火知识产权服务有限公司
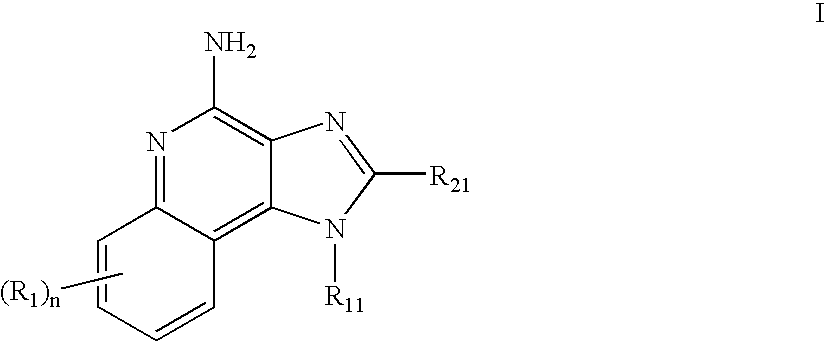
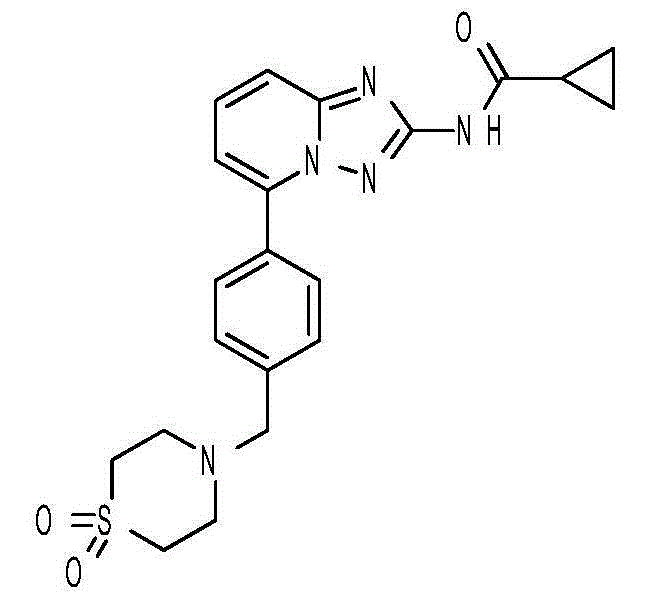
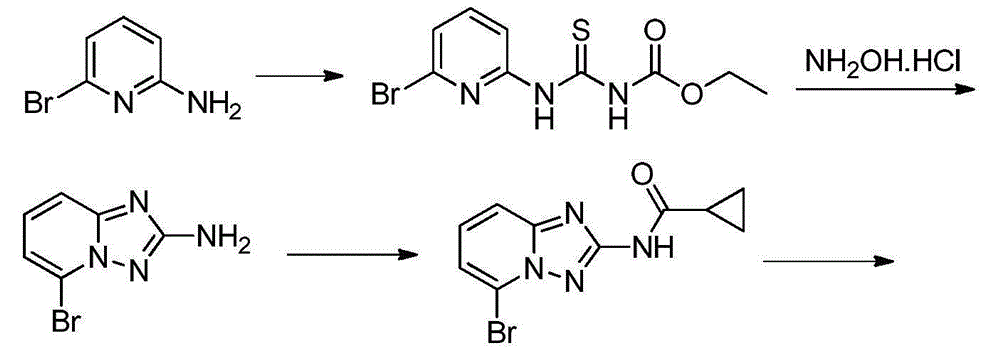
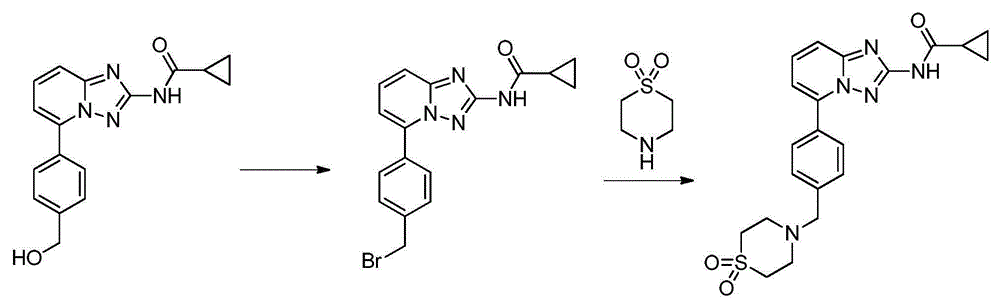
2-aminopyrido[4,3-d]pyrimidin-5-one derivatives and their use as wee-1 inhibitors
ActiveCN105209463AImprove stabilityImprove toleranceOrganic active ingredientsOrganic chemistryPyridineCancer treatment
The present invention relates to compounds of formula (I) that are useful as inhibitors of the activity of Wee-1 kinase. The present invention also relates to pharmaceutical compositions comprising these compounds and to methods of using these compounds in the treatment of cancer and methods of treating cancer.
Owner:ALMAC DISCOVERY LIMITED
Synthetic method of imidazo[1,2-a]pyridine
The invention discloses a novel method for synthesizing imidazo[1,2-a]pyridine. According to the method, imidazo[1,2-a]pyridine is synthesized from 2-aminopyridine and chalcone through oxidization cyclization by taking 1,2-dichloroethane as a solvent and iodine as a catalyst. The method has the advantages that the operation is simple, the raw materials are easily available, reaction conditions are mild, the yield is relatively high, and the practicability is strong. The method is applicable to industrial production.
Owner:NORTHWEST UNIV(CN)
Synthetic method for 3-cyano group imidazo [1, 2-a] pyridine compounds and application thereof
InactiveCN104926811AImprove universalitySimple and fast operationOrganic chemistryIodideEconomic benefits
The invention discloses a synthetic method for 3-cyano group imidazo [1, 2-a] pyridine compounds and an application thereof. Materials such as 2-aminopyridine compounds, methyl ketone compounds and benzyl cyanide are used to be reacted with N-heteroaryl nitrile through cheap and efficient cuprous iodide in catalyzing, oxidizing and cyclizing modes, and a series of the 3-cyano group imidazo [1, 2-a] pyridine compounds are constructed. The synthetic method has the advantages that the materials are easy to obtain, the operation is simple and convenient, the condition is gentle, the substrate is good in universality, the economic benefit is obtained, and the efficiency is achieved, and the method can be applied to efficient and simple and convenient composition of medicine molecules such as saripidem and necopidem.
Owner:ZHEJIANG UNIV
Injectable hydrogel capable of releasing hydrogen sulfide, and preparation method thereof
InactiveCN109666151AEasy to prepareMild reaction conditionsAerosol deliveryOintment deliveryCross-linkBiocompatibility Testing
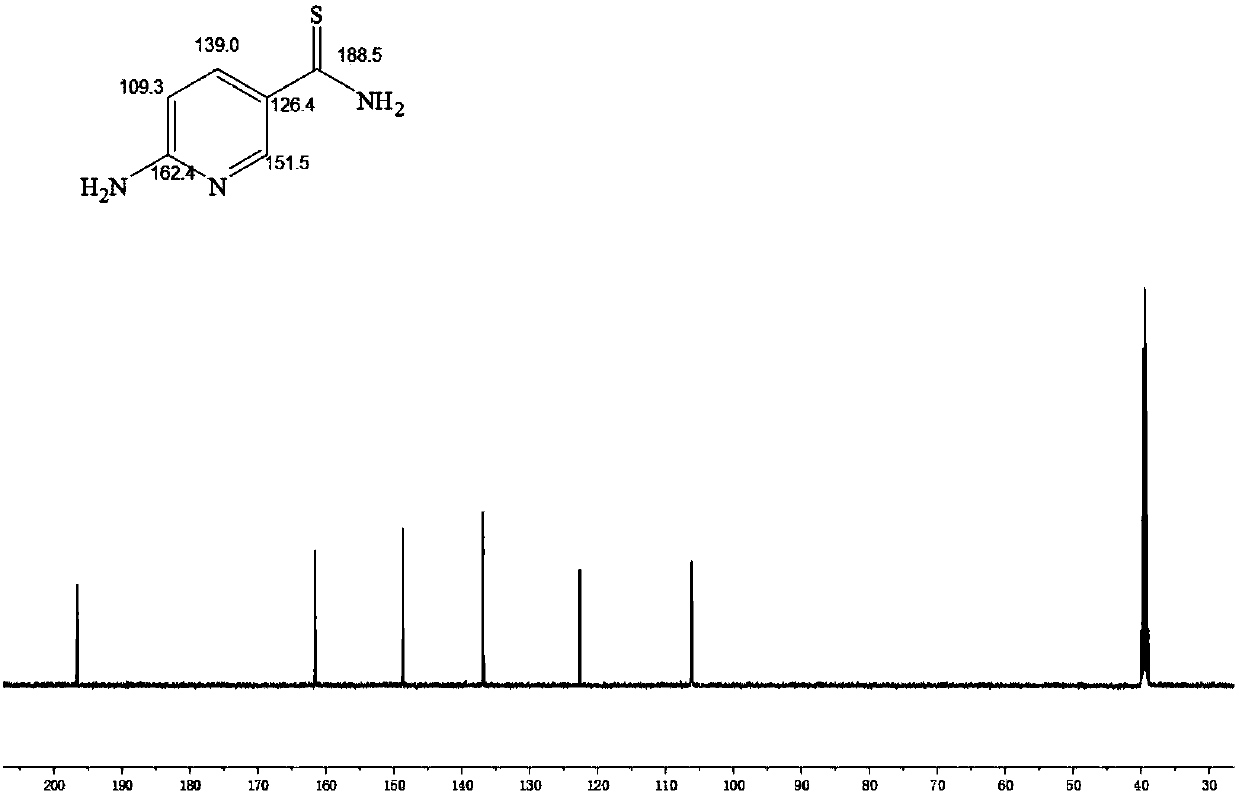
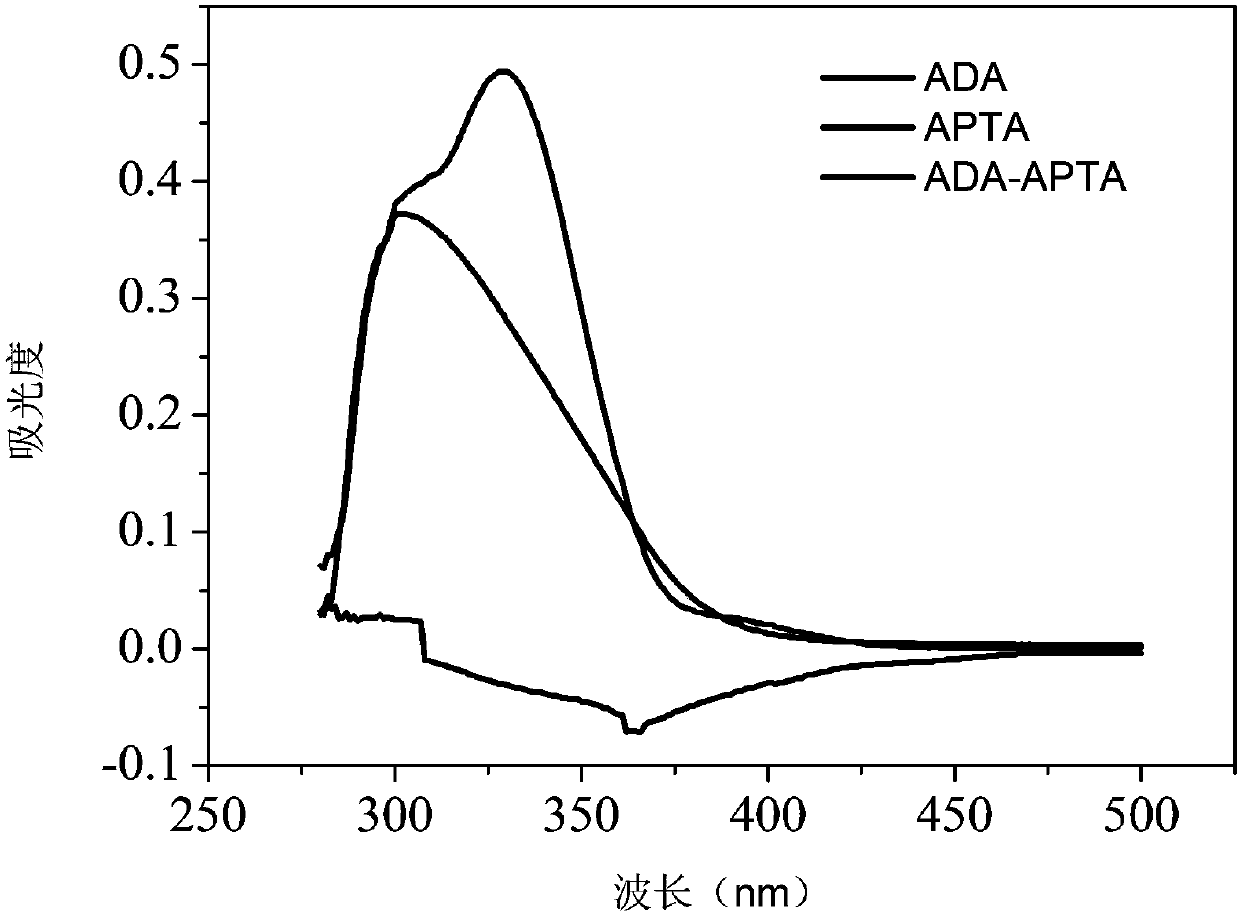
The invention provides an injectable hydrogel capable of releasing hydrogen sulfide, and a preparation method thereof. The preparation method comprises: oxidizing the hydroxyl group on sodium alginate(ALG) into an aldehyde group to obtain partially-oxidized sodium alginate (ADA), grafting 2-aminopyridine-5-thiocarboxamide (APTA) on the partially-oxidized sodium alginate by carrying out an amino reaction on the aldehyde group and the 2-aminopyridine-5-thiocarboxamide (APTA) to obtain an aldehyde group-containing macromolecular cross-linking agent partially-oxidized sodium alginate-2-aminopyridine-5-thiocarboxamide (ADA-APTA) capable of releasing hydrogen sulfide, and blending the ADA-APTA and gelatin to obtain the alginate-based injectable hydrogel capable of releasing hydrogen sulfide. According to the present invention, the preparation method is simple, the reaction condition is mild, the biocompatibility is good, and the hydrogen sulfide release amount of the gel can be adjusted bycontrolling the grafting ratio of 2-aminopyridine-5-thiocarboxamide.
Owner:TIANJIN UNIV
Preparation method of imidazopyridine compound
InactiveCN104892599AImprove adaptabilityWide adaptabilityOrganic chemistryAir atmosphereOrganic synthesis
The invention belongs to the technical field of organic chemical synthesis and discloses a preparation method of an imidazopyridine compound. The method comprises the following specific steps: adding an alkyne propyl ester compound and a 2-aminopyridine compound into an organic solvent and mixing uniformly; adding a copper salt catalyst, and stirring and reacting at 80-150 DEG C for 3-8 hours in an air atmosphere; and after the reaction is over, cooling to room temperature, performing reduced-pressure rotary evaporation and purifying through column chromatography to obtain the imidazopyridine compound. The method disclosed by the invention is safe and easy to operate, good in functional group adaptability, wide in substrate adaptability, environmentally friendly and is beneficial to industrial production, and can be widely applied to organic synthesis; the raw materials are cheap and easily available.
Owner:SOUTH CHINA UNIV OF TECH
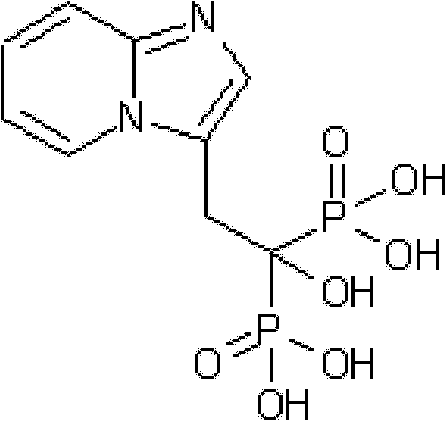
Method for preparing 1-hydroxy-2-(imidazo [1, 2-a] pyridine-3-radical) ethylidene-1, 1-bisphosphonic acid compound
InactiveCN102344463AFew reaction stepsReduce difficultyGroup 5/15 element organic compoundsCombinatorial chemistryPyridine
The invention discloses a method for preparing a 1-hydroxy-2-(imidazo [1, 2-a] pyridine-3-radical) ethylidene-1,1-bisphosphonic acid compound with high purity, which comprises the following steps of: obtaining a compound as shown in a formula (III) through nucleophilic addition of a compound (trans-4-oxygroup-2-ethyl crotonate) as shown in a formula (II) and 2-aminopyridine; hydrolyzing the compound as shown in the formula (III) under alkali condition to obtain a compound as shown in a formula (IV); and phosphorylating the compound as shown in the formula (IV) by adopting toluene as a solvent to obtain a compound as shown in a formula (I). The synthetic process condition of the product is moderate, the aftertreatment is simple, the purity is high, the reaction cost is low, and the profit is high. The industrialized production can be realized easily.
Owner:NANJING CORE TECH CO LTD
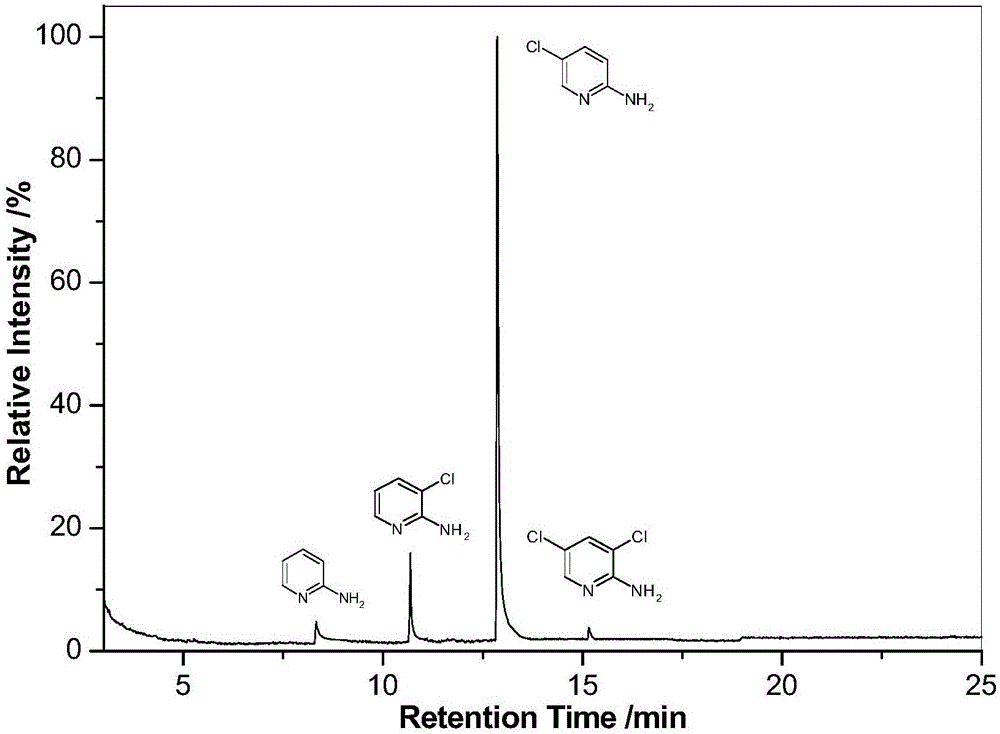
A method for preparation of 2-amino-5-chloro-pyridine
The invention provides a method for preparation of 2-amino-5-chloro-pyridine and belongs to the technical field of fine organic synthesis. 2-amino-5-chloro-pyridine is prepared by adopting 2-aminopyridine as a raw material and using hydrochloric acid and sodium hypochlorite for an oxidative chlorination reaction. The method mainly includes the following steps: at 10 DEG C, slowly and dropwise adding a certain amount of concentrated hydrochloric acid in a mixed solution of 2-aminopyridine and NaClO, conducting a reaction at constant temperature for 2 hours, increasing the temperature to 25 DEG C for continuing the reaction for 4 hours, regulating the pH of a reaction product, extracting the reaction product with dichloroethane, and conducting separation to obtain 2-amino-5-chloro-pyridine. The yield of 2-amino-5-chloro-pyridine is up to 72%. The method has the advantages that the cheap NaClO solution generated by chlorination of tail gas by chlorine gas and hydrochloric acid are used as chlorinating agents, the cost is thus reduced, and the comprehensive utilization of resources is achieved; the reaction conditions are mild, direct use of chlorine gas is avoided, safety is high, and pollution is little.
Owner:ANHUI UNIVERSITY OF TECHNOLOGY
Method for preparing 2-aminopyridine and alkyl derivative
A process for preparing 2-amino pyridine and its alkyl derivative from 2-cyanopyridine or its alkyl derivative includes incomplete hydrolysis and Hofmann degradation. Its advantage is high output rate (80%) and purity up to 99% or more.
Owner:ZHEJIANG UNIV
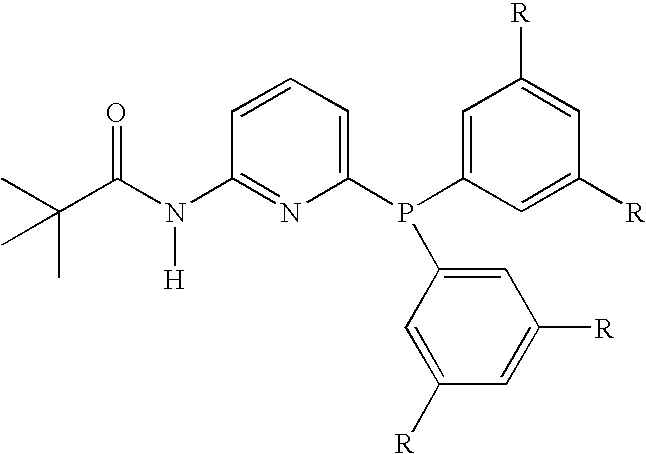
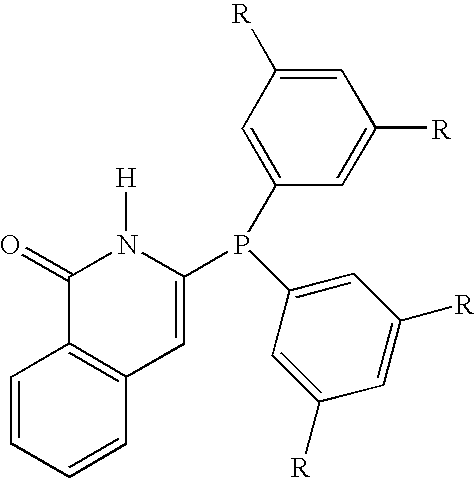
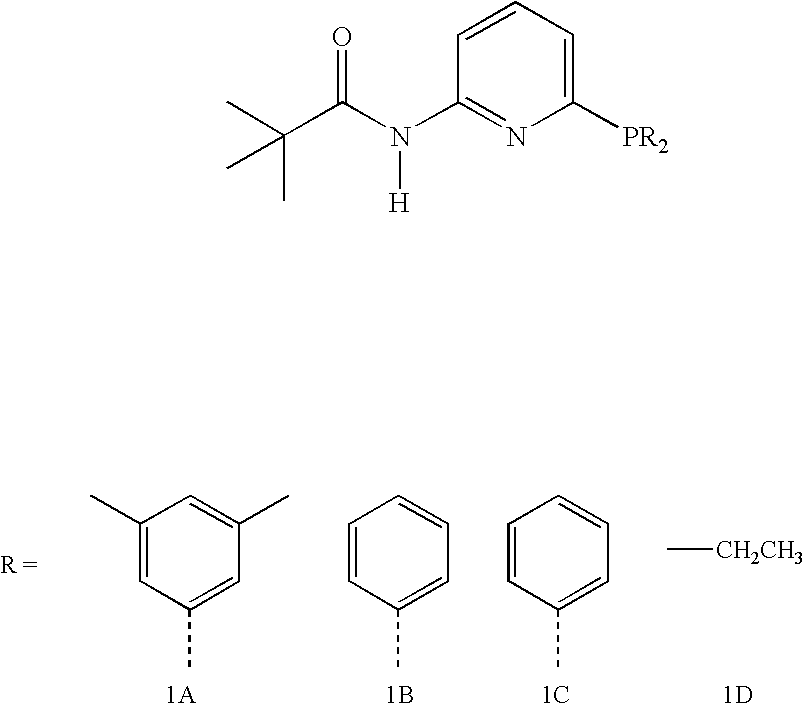
Hydroformylation process
ActiveUS7790932B1Raise the ratioOrganic compound preparationOrganic-compounds/hydrides/coordination-complexes catalystsSolventCo product
A catalyst, useful for the hydroformylation of allyl alcohol, is described. The catalyst comprises a rhodium complex and a 6-bis(3,5-dialkylphenyl)phosphino-N-pivaloyl-2-aminopyridine or a 3-bis(3,5-dialkylphenyl)phosphino-2H-isoquinolin-1-one. The invention also includes a process for the production of 4-hydroxybutyraldehyde comprising reacting allyl alcohol with a mixture of carbon monoxide and hydrogen in the presence of a solvent and the catalyst. The process gives a high ratio of the linear product 4-hydroxybutyraldehyde to the branched co-product 3-hydroxy-2-methylpropionaldehyde.
Owner:LYONDELL CHEM TECH LP
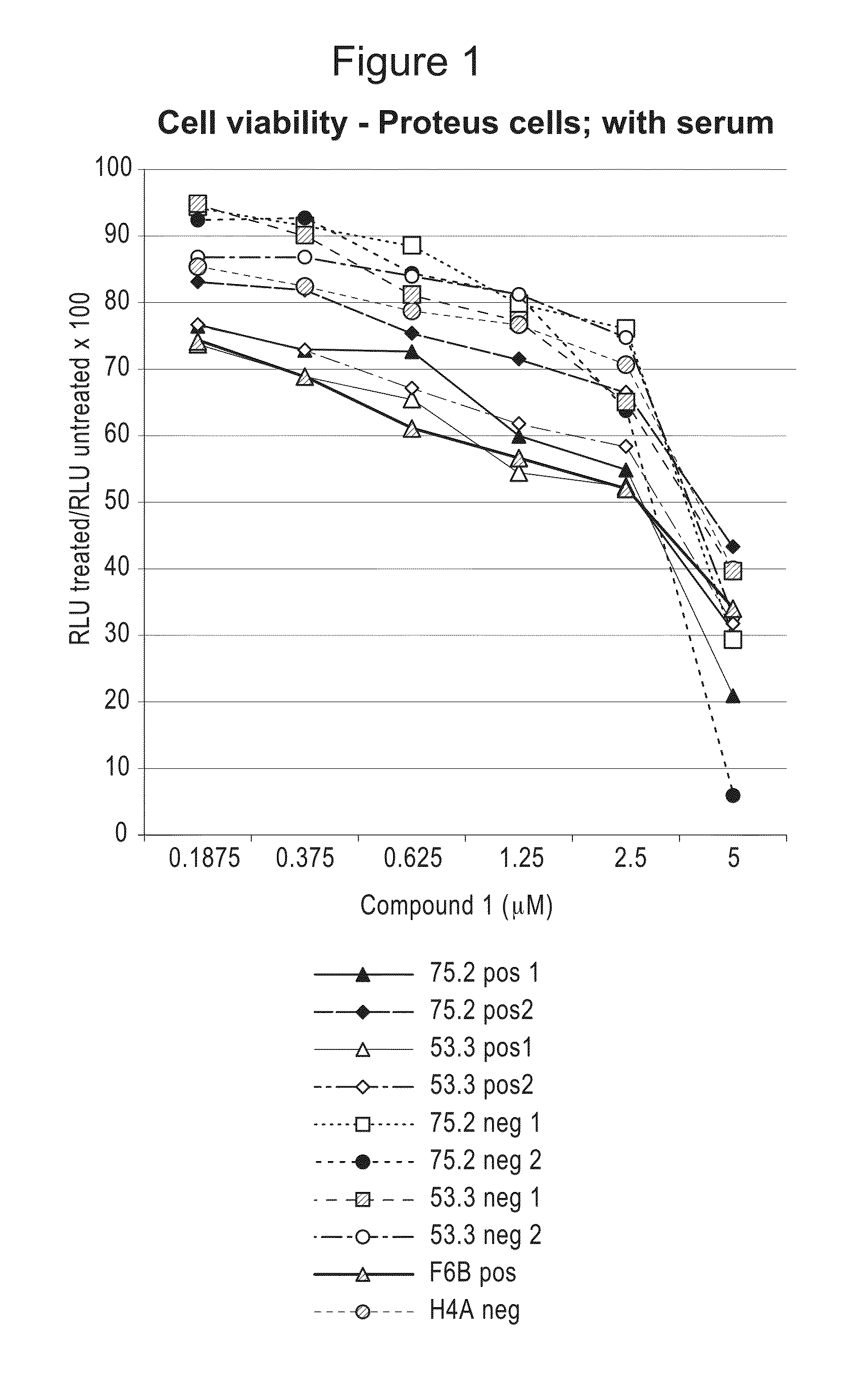
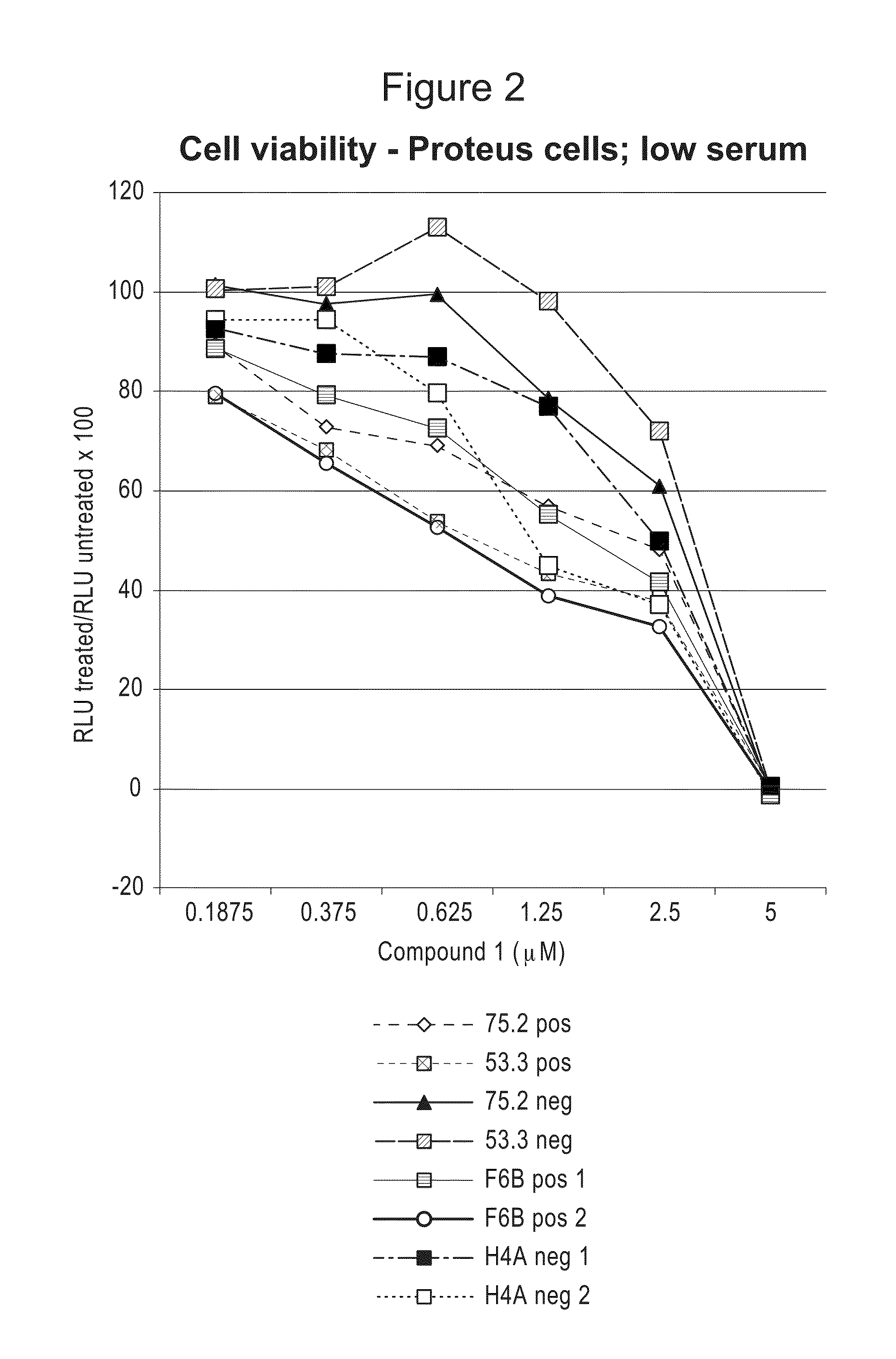
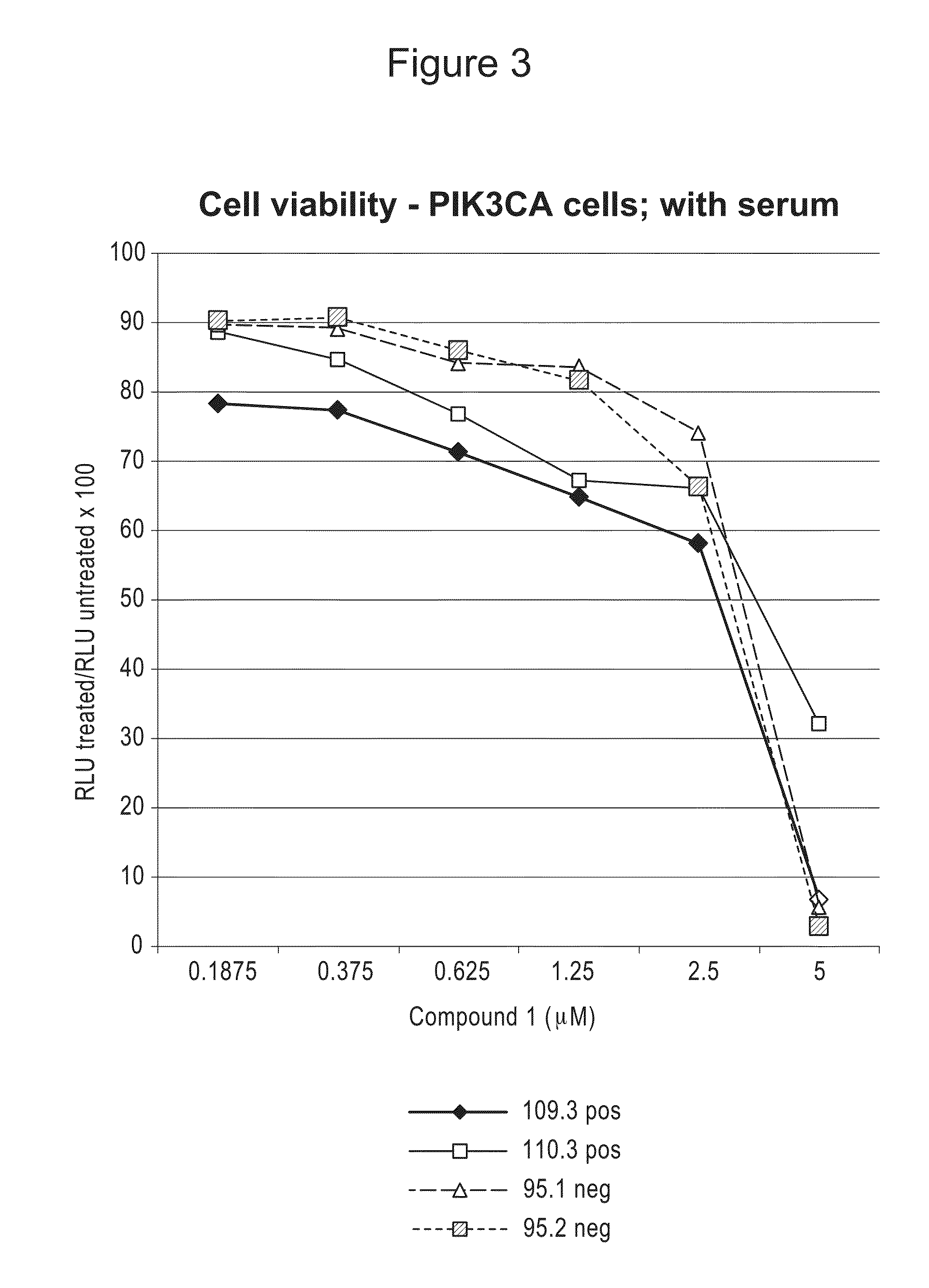
Compositions and Methods for Treating Proliferation Disorders
The present invention relates to methods of treating cell proliferative disorders, such as cancer or Proteus syndrome, by utilizing 3-(3-(4-(1-aminocyclobutyl)phenyl)-5-phenyl-3H-imidazo[4,5-b]pyridin-2-yl)pyridin-2-amine or 3-(3-(4-(1-aminocyclobutyl)phenyl)-5-(3-morpholinophenyl)-3H-imidazo[4,5-b]pyridin-2-yl)pyridin-2-amine or N-(1-(3-(3-(4-(1-aminocyclobutyl)phenyl)-2-(2-aminopyridin-3-yl)-3H-imidazo[4,5-b]pyridin-5-yl)phenyl)piperidin-4-yl)-N-methylacetamide. The methods of the present invention can also relate to methods of treating cell proliferative disorders, such as cancer or Proteus syndrome, by utilizing the above compounds in combination with ((R)-6-(2-fluorophenyl)-N-(3-(2-((2-methoxyethyl)amino)ethyl)phenyl)-5,6-dihydrobenzo[h]quinazolin-2-amine).
Owner:ARQULE INC
Electrochemical synthetic method of 3-bromine-2-phenyl-imidazo-[1,2-alpha] pyridine derivative
InactiveCN106906486AGood reaction selectivityHigh yieldElectrolysis componentsElectrolytic organic productionElectrochemistryGreen chemistry
The invention discloses an electrochemical synthetic method of a 3-bromine-2-phenyl-imidazo-[1,2-alpha] pyridine derivative. According to the method, a 2-bromoacetophenone compound and a 2-aminopyridine compound are added into DMSO containing an ammonium perchlorate electrolyte, a platinum sheet is served as the cathode, a platinum wire is served as an anode, stirring is performed at the room temperature, a constant-current reaction is performed, after the reaction is ended, reaction fluid is subjected to extraction, concentration and separation, and the 3-bromine-2-phenyl-imidazo-[1,2-alpha] pyridine derivative is obtained. Electricity is utilized to promote the reaction, an expensive metal catalyst is not needed, other oxidizing agents are not needed, heating is also not needed, the reaction can be performed at a mild room temperature, selectivity is good, and the whole process is simple and easy to perform and is consistent with the concept of green chemistry. An extra bromine source is not needed, extra oxidizing agents are also not needed, the electrochemical synthetic method is simple, efficient and wide in substrate application, and compounds with different substituent effect base groups can all achieve high yields.
Owner:SOUTH CHINA UNIV OF TECH
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com
![2-(Pyridin-2-ylamino)-pyrido[2,3-d]pyrimidin-7-ones 2-(Pyridin-2-ylamino)-pyrido[2,3-d]pyrimidin-7-ones](https://images-eureka.patsnap.com/patent_img/211494ac-0ef3-4b13-a9b2-61f9ab91cee8/US06936612-20050830-C00001.png)
![2-(Pyridin-2-ylamino)-pyrido[2,3-d]pyrimidin-7-ones 2-(Pyridin-2-ylamino)-pyrido[2,3-d]pyrimidin-7-ones](https://images-eureka.patsnap.com/patent_img/211494ac-0ef3-4b13-a9b2-61f9ab91cee8/US06936612-20050830-C00002.png)
![2-(Pyridin-2-ylamino)-pyrido[2,3-d]pyrimidin-7-ones 2-(Pyridin-2-ylamino)-pyrido[2,3-d]pyrimidin-7-ones](https://images-eureka.patsnap.com/patent_img/211494ac-0ef3-4b13-a9b2-61f9ab91cee8/US06936612-20050830-C00003.png)
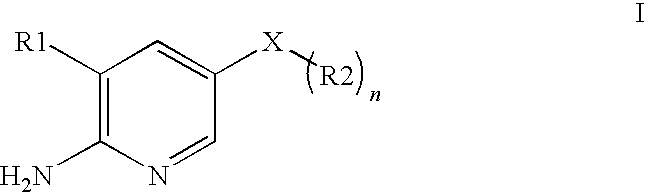
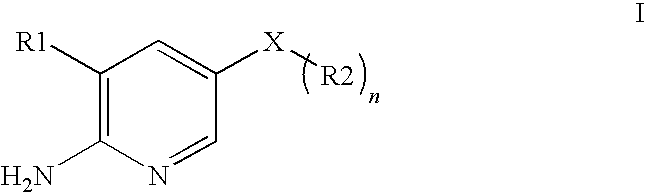
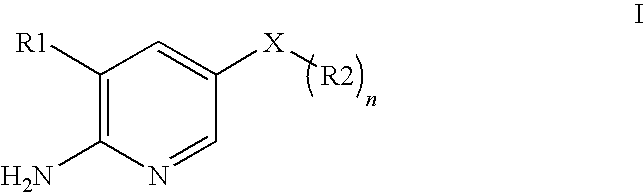
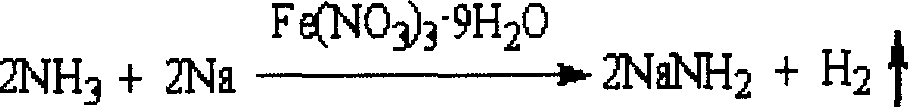

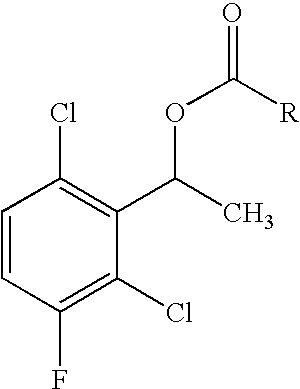
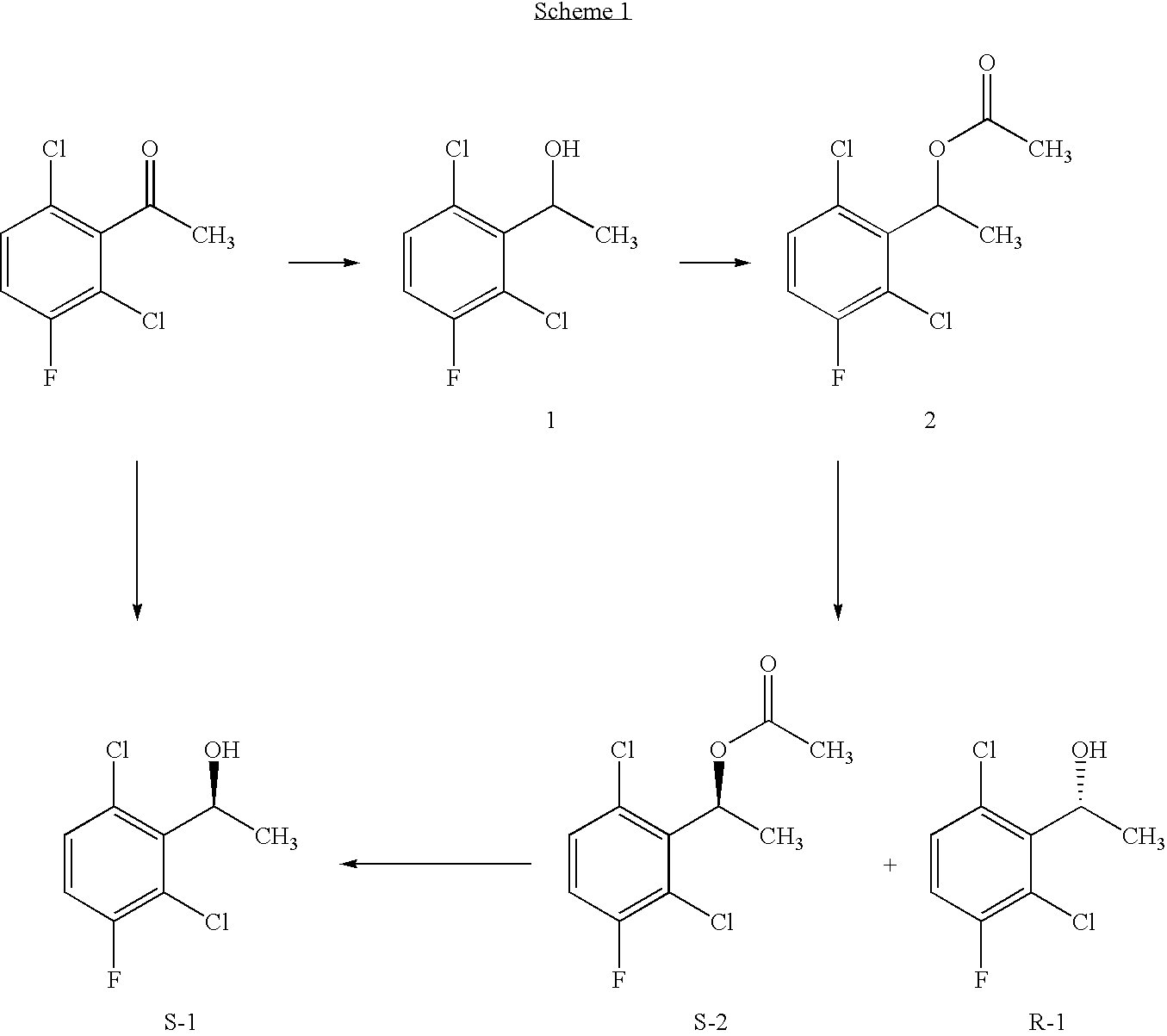
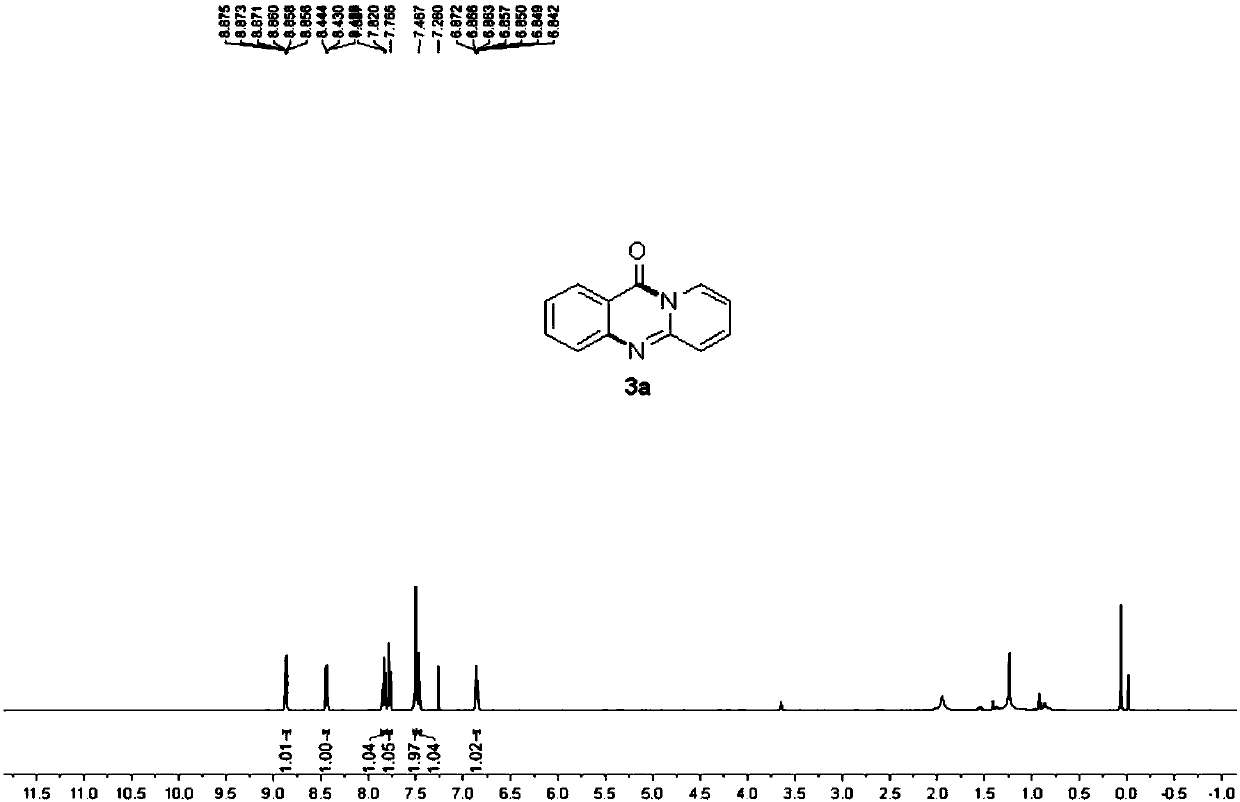
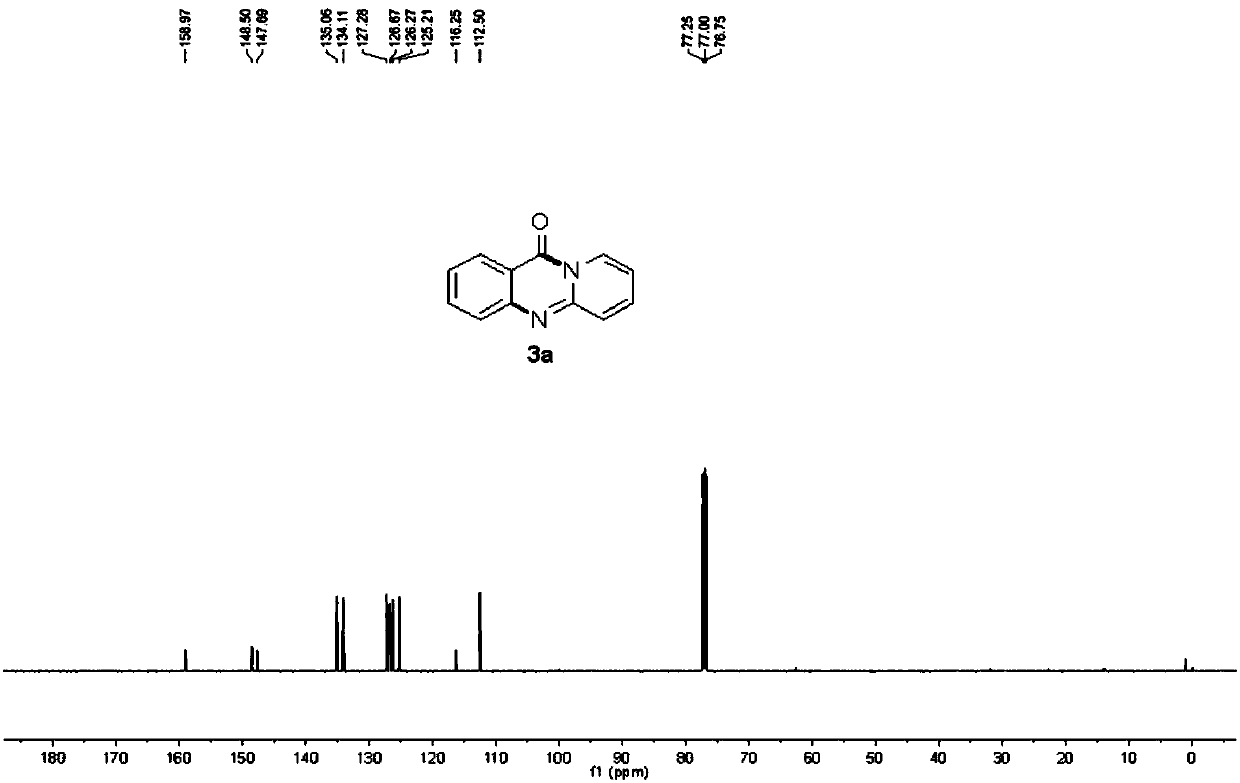
![Method for preparing 2-[imidazo[1,2-a]pyridine-3-yl]acetic acid Method for preparing 2-[imidazo[1,2-a]pyridine-3-yl]acetic acid](https://images-eureka.patsnap.com/patent_img/d95228d2-e0a0-422d-ad7c-d5c58d8b3ed6/BSA00000334200700013.PNG)
![Method for preparing 2-[imidazo[1,2-a]pyridine-3-yl]acetic acid Method for preparing 2-[imidazo[1,2-a]pyridine-3-yl]acetic acid](https://images-eureka.patsnap.com/patent_img/d95228d2-e0a0-422d-ad7c-d5c58d8b3ed6/BSA00000334200700021.PNG)
![Method for preparing 2-[imidazo[1,2-a]pyridine-3-yl]acetic acid Method for preparing 2-[imidazo[1,2-a]pyridine-3-yl]acetic acid](https://images-eureka.patsnap.com/patent_img/d95228d2-e0a0-422d-ad7c-d5c58d8b3ed6/BSA00000334200700022.PNG)
![2-(Pyridin-2-ylamino)-pyrido [2,3-D]pyrimidin-7-ones 2-(Pyridin-2-ylamino)-pyrido [2,3-D]pyrimidin-7-ones](https://images-eureka.patsnap.com/patent_img/246daac9-af8e-4f53-b4f8-c5fff883f89c/US20050137214A1-20050623-C00001.png)
![2-(Pyridin-2-ylamino)-pyrido [2,3-D]pyrimidin-7-ones 2-(Pyridin-2-ylamino)-pyrido [2,3-D]pyrimidin-7-ones](https://images-eureka.patsnap.com/patent_img/246daac9-af8e-4f53-b4f8-c5fff883f89c/US20050137214A1-20050623-C00002.png)
![2-(Pyridin-2-ylamino)-pyrido [2,3-D]pyrimidin-7-ones 2-(Pyridin-2-ylamino)-pyrido [2,3-D]pyrimidin-7-ones](https://images-eureka.patsnap.com/patent_img/246daac9-af8e-4f53-b4f8-c5fff883f89c/US20050137214A1-20050623-C00003.png)
![2-aminopyrido[4,3-d]pyrimidin-5-one derivatives and their use as wee-1 inhibitors 2-aminopyrido[4,3-d]pyrimidin-5-one derivatives and their use as wee-1 inhibitors](https://images-eureka.patsnap.com/patent_img/efee35d8-c78c-4038-b3b6-d57128353e70/BDA0000845207990000041.PNG)
![2-aminopyrido[4,3-d]pyrimidin-5-one derivatives and their use as wee-1 inhibitors 2-aminopyrido[4,3-d]pyrimidin-5-one derivatives and their use as wee-1 inhibitors](https://images-eureka.patsnap.com/patent_img/efee35d8-c78c-4038-b3b6-d57128353e70/BDA0000845207990000051.PNG)
![2-aminopyrido[4,3-d]pyrimidin-5-one derivatives and their use as wee-1 inhibitors 2-aminopyrido[4,3-d]pyrimidin-5-one derivatives and their use as wee-1 inhibitors](https://images-eureka.patsnap.com/patent_img/efee35d8-c78c-4038-b3b6-d57128353e70/BDA0000845207990000061.PNG)
![Synthetic method of imidazo[1,2-a]pyridine Synthetic method of imidazo[1,2-a]pyridine](https://images-eureka.patsnap.com/patent_img/0c7394e3-6ca5-45dd-9ba3-5222d6765aa3/226696DEST_PATH_IMAGE001.PNG)
![Synthetic method of imidazo[1,2-a]pyridine Synthetic method of imidazo[1,2-a]pyridine](https://images-eureka.patsnap.com/patent_img/0c7394e3-6ca5-45dd-9ba3-5222d6765aa3/369819DEST_PATH_IMAGE004.PNG)
![Synthetic method of imidazo[1,2-a]pyridine Synthetic method of imidazo[1,2-a]pyridine](https://images-eureka.patsnap.com/patent_img/0c7394e3-6ca5-45dd-9ba3-5222d6765aa3/442817DEST_PATH_IMAGE001.PNG)
![Synthetic method for 3-cyano group imidazo [1, 2-a] pyridine compounds and application thereof Synthetic method for 3-cyano group imidazo [1, 2-a] pyridine compounds and application thereof](https://images-eureka.patsnap.com/patent_img/45f3da4a-19ff-4e24-b23a-0e6c4c4b5110/BDA0000739945350000011.PNG)
![Synthetic method for 3-cyano group imidazo [1, 2-a] pyridine compounds and application thereof Synthetic method for 3-cyano group imidazo [1, 2-a] pyridine compounds and application thereof](https://images-eureka.patsnap.com/patent_img/45f3da4a-19ff-4e24-b23a-0e6c4c4b5110/BDA0000739945350000021.PNG)
![Synthetic method for 3-cyano group imidazo [1, 2-a] pyridine compounds and application thereof Synthetic method for 3-cyano group imidazo [1, 2-a] pyridine compounds and application thereof](https://images-eureka.patsnap.com/patent_img/45f3da4a-19ff-4e24-b23a-0e6c4c4b5110/BDA0000739945350000022.PNG)
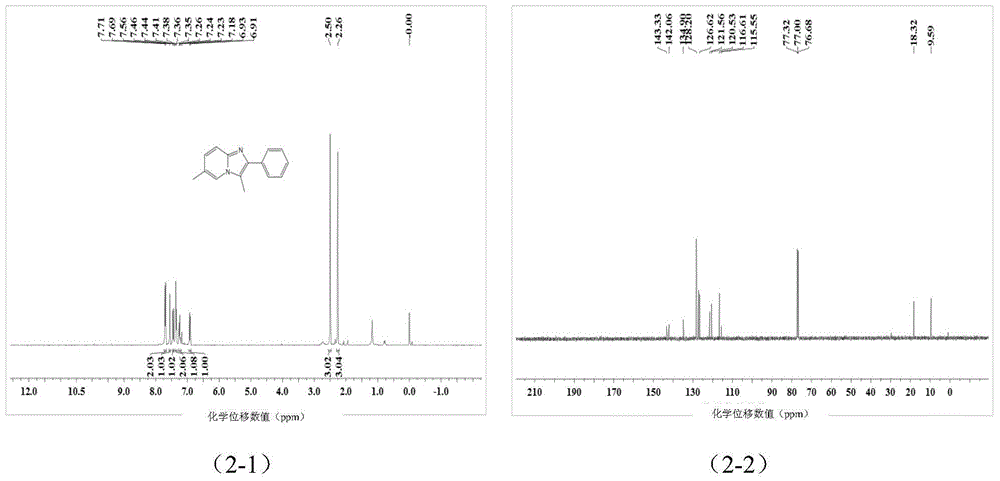
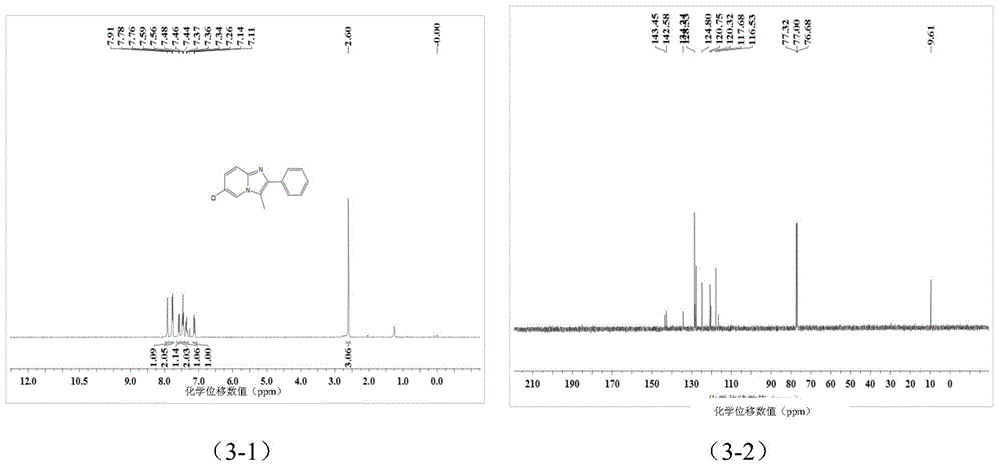
![Method for preparing 1-hydroxy-2-(imidazo [1, 2-a] pyridine-3-radical) ethylidene-1, 1-bisphosphonic acid compound Method for preparing 1-hydroxy-2-(imidazo [1, 2-a] pyridine-3-radical) ethylidene-1, 1-bisphosphonic acid compound](https://images-eureka.patsnap.com/patent_img/accdd967-c371-4f38-b159-a4c95fd749fb/FSA00000592507100011.png)
![Method for preparing 1-hydroxy-2-(imidazo [1, 2-a] pyridine-3-radical) ethylidene-1, 1-bisphosphonic acid compound Method for preparing 1-hydroxy-2-(imidazo [1, 2-a] pyridine-3-radical) ethylidene-1, 1-bisphosphonic acid compound](https://images-eureka.patsnap.com/patent_img/accdd967-c371-4f38-b159-a4c95fd749fb/FSA00000592507100012.png)
![Method for preparing 1-hydroxy-2-(imidazo [1, 2-a] pyridine-3-radical) ethylidene-1, 1-bisphosphonic acid compound Method for preparing 1-hydroxy-2-(imidazo [1, 2-a] pyridine-3-radical) ethylidene-1, 1-bisphosphonic acid compound](https://images-eureka.patsnap.com/patent_img/accdd967-c371-4f38-b159-a4c95fd749fb/FSA00000592507100013.png)
![Electrochemical synthetic method of 3-bromine-2-phenyl-imidazo-[1,2-alpha] pyridine derivative Electrochemical synthetic method of 3-bromine-2-phenyl-imidazo-[1,2-alpha] pyridine derivative](https://images-eureka.patsnap.com/patent_img/73498aee-0784-4d80-916a-62907d956d9e/HDA0001230545540000011.png)
![Electrochemical synthetic method of 3-bromine-2-phenyl-imidazo-[1,2-alpha] pyridine derivative Electrochemical synthetic method of 3-bromine-2-phenyl-imidazo-[1,2-alpha] pyridine derivative](https://images-eureka.patsnap.com/patent_img/73498aee-0784-4d80-916a-62907d956d9e/HDA0001230545540000012.png)
![Electrochemical synthetic method of 3-bromine-2-phenyl-imidazo-[1,2-alpha] pyridine derivative Electrochemical synthetic method of 3-bromine-2-phenyl-imidazo-[1,2-alpha] pyridine derivative](https://images-eureka.patsnap.com/patent_img/73498aee-0784-4d80-916a-62907d956d9e/HDA0001230545540000021.png)