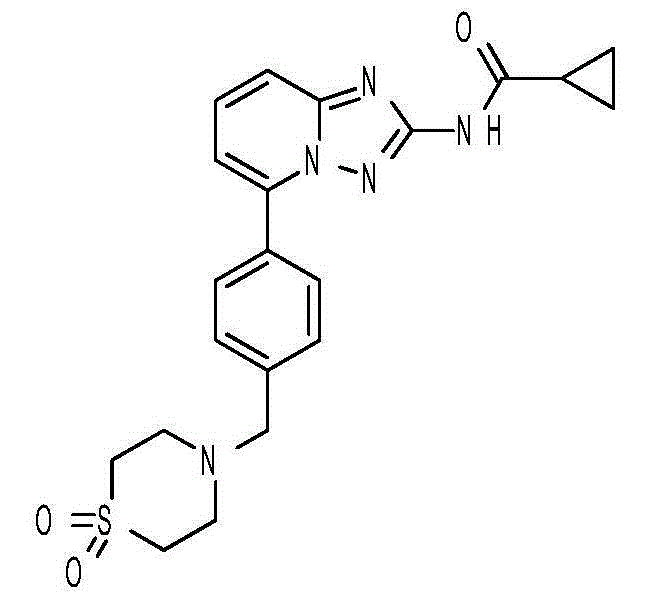
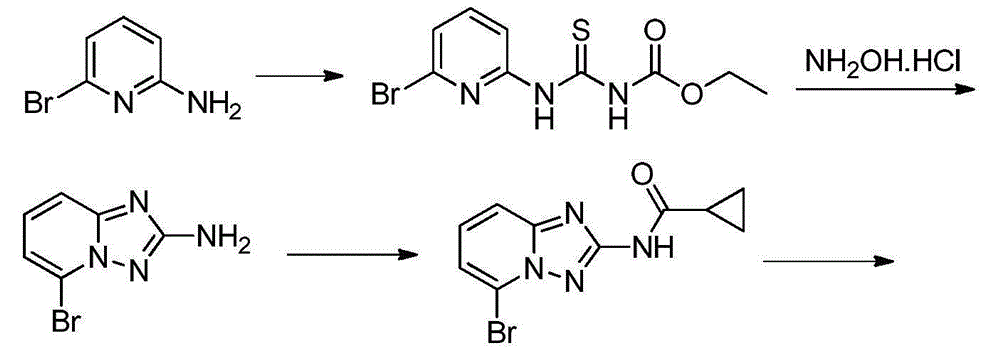
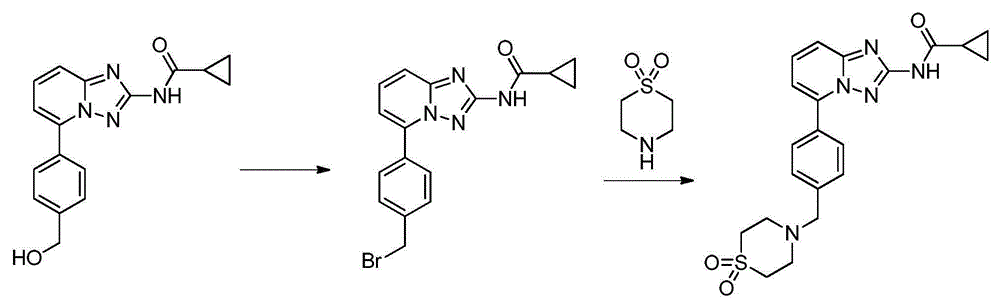
Filgotinib synthetic method
A synthesis method and condensation technology, applied in the direction of organic chemistry, etc., can solve the problems of intermediate products and final products containing many impurities and by-products, unfavorable industrial production promotion, cumbersome operation, etc., achieve high yield, simplify operation, Reasonable effect of technical solutions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029]A) Preparation of 6-hydroxyl-2-tert-butoxycarbonylaminopyridine: first carry out condensation reaction by 6-chloro-2-aminopyridine and di-tert-butyl dicarbonate in dichloromethane under stirring, 6-chloro-2-aminopyridine The molar ratio of 2-aminopyridine, dichloromethane and di-tert-butyl dicarbonate is 1:10:1.2, the temperature of the condensation reaction is 60°C, and the time of the condensation reaction is 3h. The TLC spot plate confirms that the reaction is complete, and the reaction The liquid was concentrated to dryness by rotary evaporation to obtain 6-chloro-2-tert-butoxycarbonylaminopyridine, and then 6-chloro-2-tert-butoxycarbonylaminopyridine was dropped into a sodium hydroxide solution with a concentration of 9.9% by mass percentage, In a system composed of tetrabutylammonium chloride, 1,4-dioxane and water, the hydrolysis reaction is carried out under stirring, 6-chloro-2-tert-butoxycarbonylaminopyridine, the concentration of which is 9.9% by mass The mola...
Embodiment 2
[0042] A) Preparation of 6-hydroxyl-2-tert-butoxycarbonylaminopyridine: first carry out condensation reaction by 6-chloro-2-aminopyridine and di-tert-butyl dicarbonate in dichloroethane under stirring, 6-chloro - The molar ratio of 2-aminopyridine, dichloroethane and di-tert-butyl dicarbonate is 1:5:1.3, the temperature of the condensation reaction is 10°C, the time of the condensation reaction is 8h, and the TLC spot plate confirms that the reaction is complete , the reaction solution was concentrated to dryness by rotary evaporation to obtain 6-chloro-2-tert-butoxycarbonylaminopyridine, and then 6-chloro-2-tert-butoxycarbonylaminopyridine was dropped into potassium hydroxide with a mass percentage concentration of 20%. Solution, benzyltriethylammonium bromide, p-xylene and water constitute the system and carry out the hydrolysis reaction under stirring, 6-chloro-2-tert-butoxycarbonylaminopyridine, mass percent concentration of 20% hydrogen The molar ratio of potassium oxide ...
Embodiment 3
[0049] A) Preparation of 6-hydroxyl-2-tert-butoxycarbonylaminopyridine: Condensation reaction of 6-chloro-2-aminopyridine in 1,4-dioxane with di-tert-butyl dicarbonate under stirring , the molar ratio of 6-chloro-2-aminopyridine, 1,4-dioxane and di-tert-butyl dicarbonate is 1:30:1.5, the condensation reaction temperature is 80°C, and the condensation reaction time is After 0.5h, the TLC spot plate confirmed that the reaction was complete, and the reaction solution was concentrated to dryness by rotary evaporation to obtain 6-chloro-2-tert-butoxycarbonylaminopyridine, and then 6-chloro-2-tert-butoxycarbonylaminopyridine was put into the mass In a system composed of 33.4% cesium hydroxide solution, tetradecyltrimethylammonium chloride, N,N-2 methylformamide and water and under stirring, the hydrolysis reaction, 6-chloro- The molar ratio of 2-tert-butoxycarbonylaminopyridine, 33.4% cesium hydroxide solution, tetradecyltrimethylammonium chloride, N,N-2 methylformamide and water is...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com