Injectable hydrogel capable of releasing hydrogen sulfide, and preparation method thereof
A technology of hydrogen sulfide and water for injection, which is applied in the direction of pharmaceutical formulations, active ingredients of heterocyclic compounds, non-active ingredients of polymer compounds, etc., can solve problems such as uncontrollable processes, achieve good biocompatibility, mild reaction conditions, and easy preparation The effect of simple method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
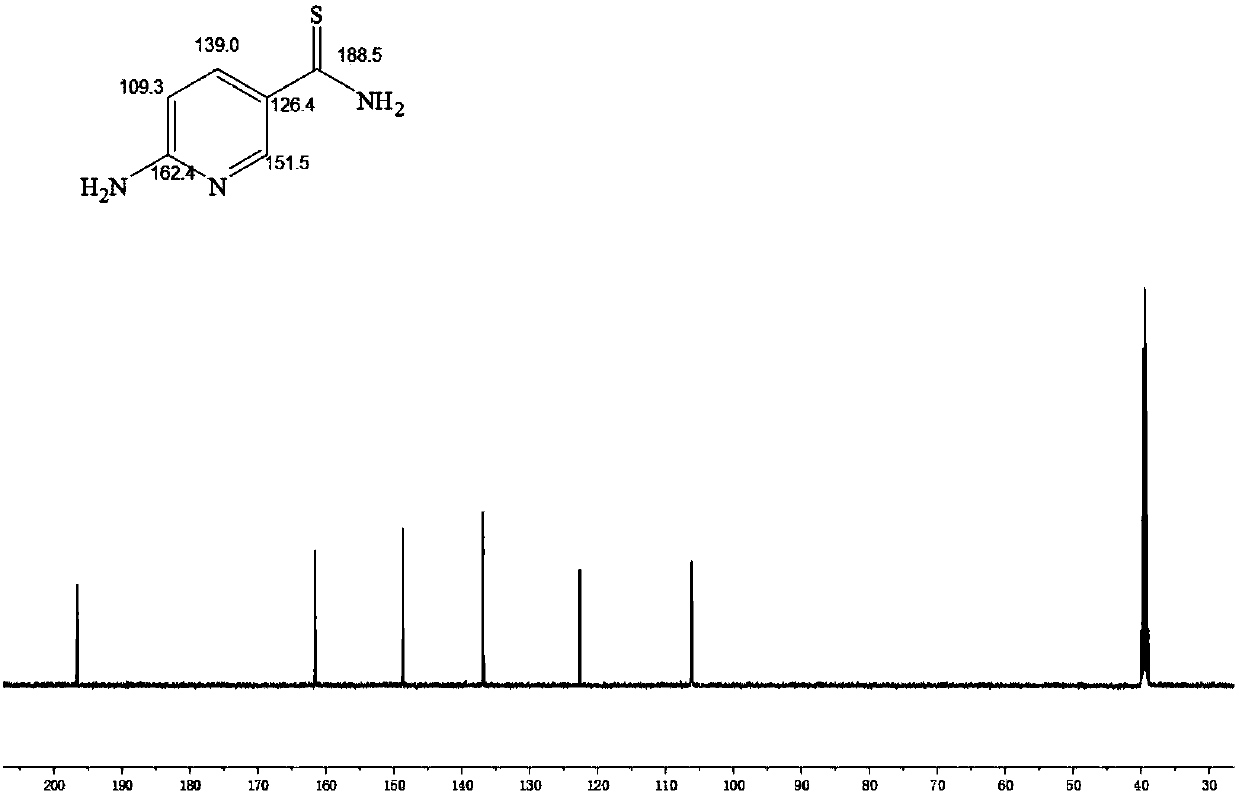
[0040] Taking the degree of oxidation as 50% and the ratio of 2-aminopyridine-5-thiocarboxamide as 10% as an example, the preparation of alginate-based injectable hydrogel that can release hydrogen sulfide is illustrated.
[0041] Weigh 5.0 g of sodium alginate and add it to 25 ml of absolute ethanol, and stir until it is evenly dispersed. Weigh 2.67g of sodium periodate, add it to 25ml of deionized water, and stir until completely dissolved in the dark (sodium periodate is easy to decompose when exposed to light, so it needs to be stirred in the dark, and the reaction should be carried out in the dark). Pour sodium periodate into the suspension of sodium alginate and react at room temperature for 4 hours. After 4h, 5ml of ethylene glycol was added to terminate the reaction for 15min. Pour the reaction solution into a dialysis bag, and perform dialysis with deionized water for three days, and change the dialysis water every day, about 3-4 times a day. The dialysis-completed pr...
Embodiment 2
[0045] Taking the degree of oxidation of sodium alginate as 50% and the ratio of 2-aminopyridine-5-thiocarboxamide as 20% as an example, the preparation of alginate-based injectable hydrogel that can release hydrogen sulfide is illustrated.
[0046] Weigh 5.0 g of sodium alginate and add it to 25 ml of absolute ethanol, and stir until it is evenly dispersed. Weigh 2.67g of sodium periodate, add it to 25ml of deionized water, and stir until completely dissolved in the dark (sodium periodate is easy to decompose when exposed to light, so it needs to be stirred in the dark, and the reaction should be carried out in the dark). Pour sodium periodate into the suspension of sodium alginate and react at room temperature for 4 hours. After 4h, 5ml of ethylene glycol was added to terminate the reaction for 15min. Pour the reaction solution into a dialysis bag, and perform dialysis with deionized water for three days, and change the dialysis water every day, about 3-4 times a day. The ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com