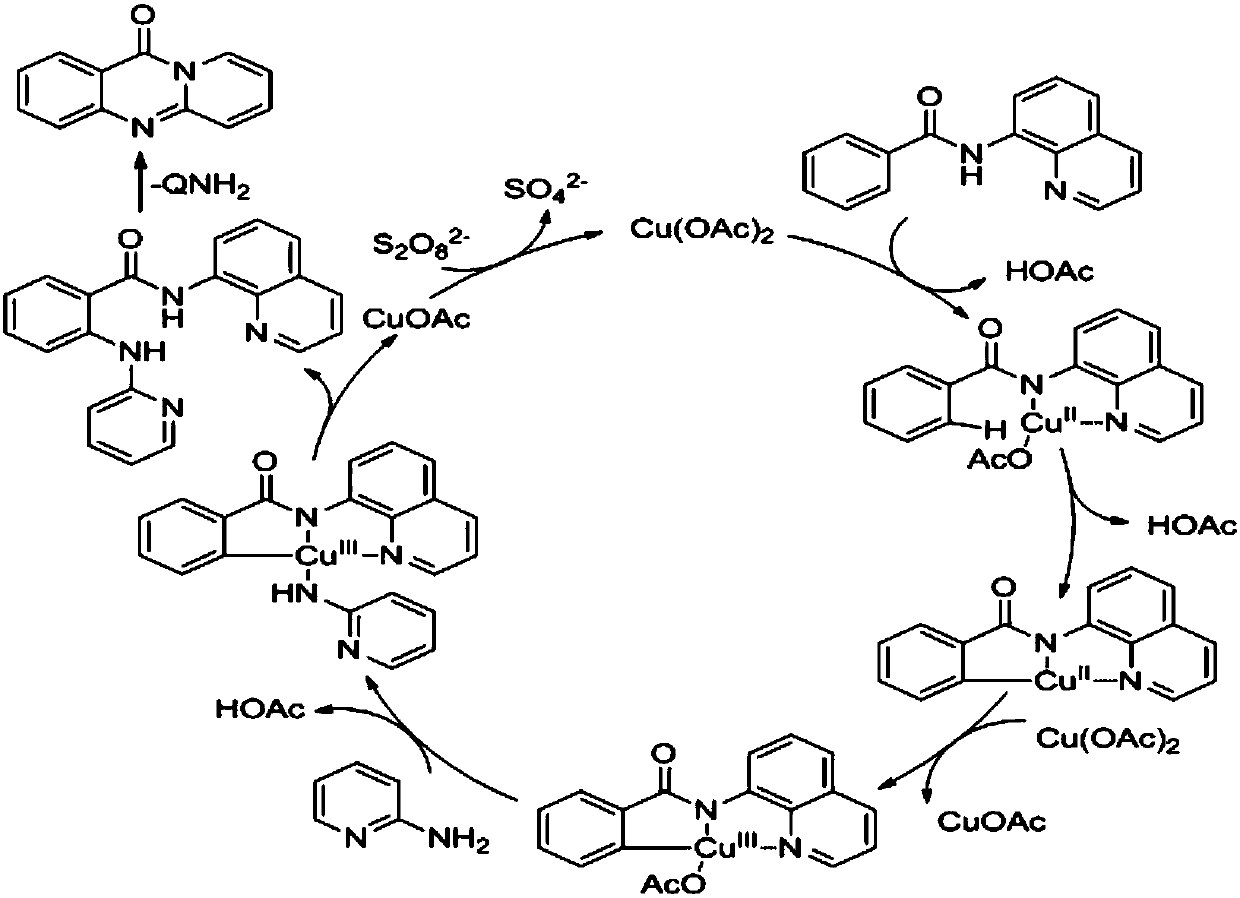
Method for preparing pyridinoquinazolinone compound through catalysis of copper compound
A technology of pyridoquinazolone and copper compound, which is applied in the field of organic synthesis and metal catalysis, can solve the problems of unavailable and expensive, and achieve the effects of high yield, simple reaction operation, and the development of industrial production
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
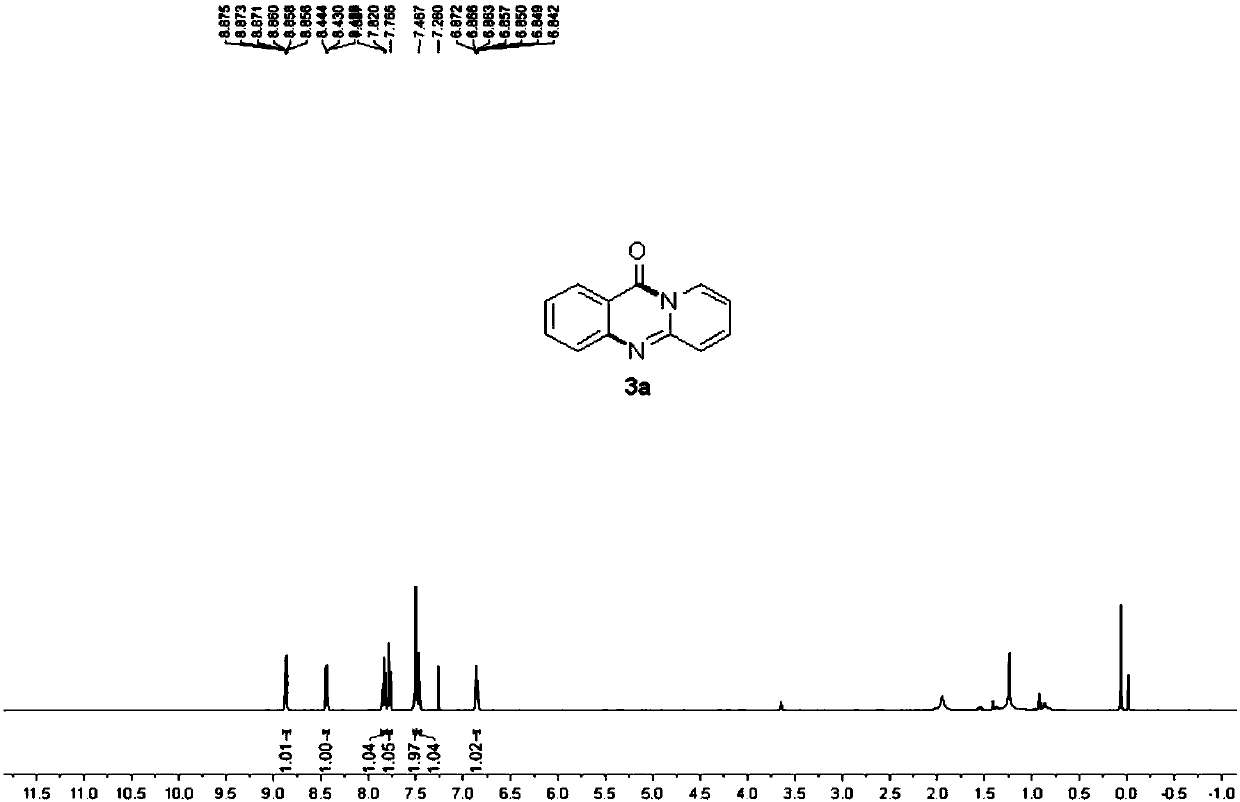
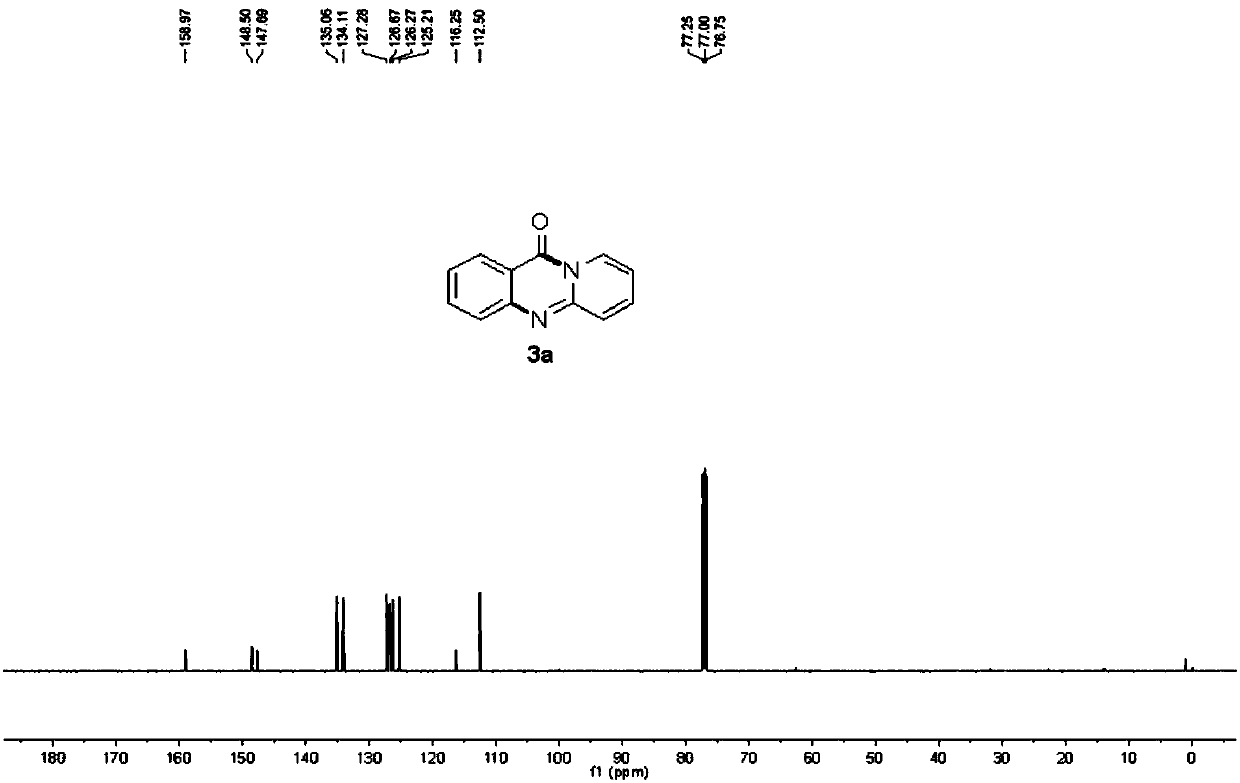
[0051] Example 1: 11H-pyrido[2,1-b]quinazolin-11-one
[0052] 248mg (1mmol) N- (quinolin-8-yl) benzamide and 188mg (2mmol) 2-aminopyridine, 18mg (0.1mmol) catalyst Cu (OAc) 2 ,540mg (2mmol) Oxidant K 2 S 2 o 8 , 3mL solvent dimethyl sulfoxide (DMSO) was added to a 30mL sealed tube under air. Then the sealed tube was placed in an oil bath at 100°C for 4 h. After the reaction was finished, the reaction solution was cooled to room temperature, 80 mL of ethyl acetate was added, and the column chromatography (ethyl acetate was used as eluent) combined the organic layers, and the organic layers were washed with water, dried over anhydrous sodium sulfate, filtered, and the filtrate was reduced to After pressure distillation, it was separated by silica gel column chromatography (ethyl acetate: petroleum ether = 1:3 as eluent) to obtain 163 mg of yellow solid with a yield of 83%.
[0053] The various characterization data of the resulting product are as follows:
[0054] M.p.=208...
Embodiment 2
[0067] Example 2: 3-Methyl-11H-pyrido[2,1-b]quinazolin-11-one
[0068] With 262mg (1mmol) 4-methyl-N-(quinolin-8-yl) benzamide and 188mg (2mmol) 2-aminopyridine, 18mg (0.1mmol) catalyst Cu(OAc) 2 ,540mg (2mmol) Oxidant K 2 S 2 o 8 , 3mL solvent dimethyl sulfoxide (DMSO) was added to a 30mL sealed tube under air. Then the sealed tube was placed in an oil bath at 100°C for 4 h. After the reaction was finished, the reaction solution was cooled to room temperature, 80 mL of ethyl acetate was added, and the column chromatography (ethyl acetate was used as eluent) combined the organic layers, and the organic layers were washed with water, dried over anhydrous sodium sulfate, filtered, and the filtrate was reduced to After pressure distillation, it was separated by silica gel column chromatography (ethyl acetate / petroleum ether: 1 / 3 as eluent) to obtain 168 mg of yellow solid with a yield of 80%.
[0069] The various characterization data of the resulting product are as follows:...
Embodiment 3
[0082] Example 3: 3-methoxy-11H-pyrido[2,1-b]quinazolin-11-one
[0083] With 278mg (1mmol) 4-methoxy-N-(quinolin-8-yl) benzamide and 188mg (2mmol) 2-aminopyridine, 18mg (0.1mmol) catalyst Cu(OAc) 2 ,540mg (2mmol) Oxidant K 2 S 2 o 8 , 3mL solvent dimethyl sulfoxide (DMSO) was added to a 30mL sealed tube under air. Then the sealed tube was placed in an oil bath at 100°C for 4 h. After the reaction was finished, the reaction solution was cooled to room temperature, 80ml of ethyl acetate was added, flash column chromatography (ethyl acetate was used as eluent), the organic layers were combined, the organic layer was washed with water, dried over anhydrous sodium sulfate, filtered, and the filtrate was reduced After pressure distillation, it was separated by silica gel column chromatography (ethyl acetate / petroleum ether: 1 / 3 as eluent) to obtain 190 mg of a yellow solid with a yield of 84%.
[0084] The various characterization data of the resulting product are as follows: ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com