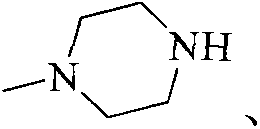
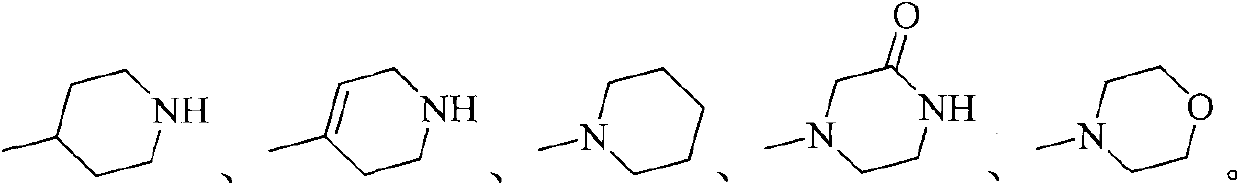
Substituted 2-aminopyridine inhibitor for protein kinase
A technology of alkyl and phenyl, which is applied in the direction of medical preparations containing active ingredients, compounds of Group 5/15 elements of the periodic table, drug combinations, etc., and can solve problems such as drug resistance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0466] Example 1: 4-(1-(3-chloro-4-fluorophenyl)-1H-pyrazol-5-yl)-5-(2-methoxy-4-(piperazin-1-yl)phenyl)- 2-aminopyridine
[0467]
[0468] General synthesis method:
[0469]
[0470] Step 1: 2-Chloro-4-(1-(3-chloro-4-fluorophenyl)-1H-pyrazol-5-yl)pyridine
[0471]
[0472] 1-(2-chloropyridin-4-yl)-3-(dimethylamino)prop-2-en-1-one (2.11g, 10mmol), 3-chloro-4-fluorophenylhydrazine (1.61g, 10mmol), a few drops of glacial acetic acid and water (1mL) were added to ethanol (50mL), refluxed for 1.5 hours, the solvent was spin-dried, ethyl acetate and water were added, extracted, the organic phase was dried, purified by silica gel column chromatography, and 2-chloro- 4-(1-(3-Chloro-4-fluorophenyl)-1H-pyrazol-5-yl)pyridine (2.50 g), yield: 81%. MS m / z [ESI]: 308.0 [M+1].
[0473] Step 2: 4-(1-(3-Chloro-4-fluorophenyl)-1H-pyrazol-5-yl)-N-(dibenzylidene)-2-aminopyridine
[0474]
[0475] 2-Chloro-4-(1-(3-chloro-4-fluorophenyl)-1H-pyrazol-5-yl)pyridine ((2.0g, 6.5mmol)...
Embodiment 2
[0488] Example 2: 4-(1-(4-fluorophenyl)-1H-pyrazol-5-yl)-5-(2-methoxy-4-(piperazin-1-yl)phenyl)- 2-aminopyridine hydrochloride
[0489]
[0490] Step 1: 2-Chloro-4-(1-(4-fluorophenyl)-1H-pyrazol-5-yl)pyridine
[0491]
[0492] Referring to the method of step 1 in Example 1, 4-fluorophenylhydrazine was used instead of 3-chloro-4-fluorophenylhydrazine to obtain the target compound with a yield of 86%. MS m / z [ESI]: 274.0 [M+1].
[0493] Step 2: 4-(1-(4-Fluorophenyl)-1H-pyrazol-5-yl)-N-(dibenzylidene)-2-aminopyridine
[0494]
[0495] With reference to the method of step 2 in Example 1, replace 2-chloro-4-(1-(3 -Chloro-4-fluorophenyl)-1H-pyrazol-5-yl)pyridine to obtain the target compound, yield: 56%. MS m / z [ESI]: 419.2 [M+1].
[0496] Step 3: 4-(1-(4-fluorophenyl)-1H-pyrazol-5-yl)-2-aminopyridine
[0497]
[0498] Referring to the method of step 3 in Example 1, replace 4 with 4-(1-(4-fluorophenyl)-1H-pyrazol-5-yl)-N-(dibenzylidene)-2-aminopyridine -(1-(3-chlo...
Embodiment 3
[0508] Example 3: 4-(1-(3-fluorophenyl)-1H-pyrazol-5-yl)-5-(2-methoxy-4-(piperazin-1-yl)phenyl)- 2-aminopyridine hydrochloride
[0509]
[0510] Step 1: 2-Chloro-4-(1-(3-fluorophenyl)-1H-pyrazol-5-yl)pyridine
[0511]
[0512] Referring to the method of step 1 in Example 1, using 3-fluorophenylhydrazine instead of 3-chloro-4-fluorophenylhydrazine, the target compound was obtained with a yield of 79%. MS m / z [ESI]: 274.0 [M+1].
[0513] Step 2: 4-(1-(3-Fluorophenyl)-1H-pyrazol-5-yl)-N-(dibenzylidene)-2-aminopyridine
[0514]
[0515] With reference to the method of step 2 in Example 1, replace 2-chloro-4-(1-(3 -Chloro-4-fluorophenyl)-1H-pyrazol-5-yl)pyridine to obtain the target compound, yield: 69%. MS m / z [ESI]: 419.2 [M+1].
[0516]Step 3: 4-(1-(3-Fluorophenyl)-1H-pyrazol-5-yl)-2-aminopyridine
[0517]
[0518] Referring to the method of step 3 in Example 1, replace 4 with 4-(1-(3-fluorophenyl)-1H-pyrazol-5-yl)-N-(dibenzylidene)-2-aminopyridine -(1-(3-chlo...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com