Preparation method of imidazopyridine compound
A technology for imidazopyridine and compound is applied in the field of preparation of imidazopyridine compounds, and achieves the effects of safe and simple operation, good adaptability and simple and easy method.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
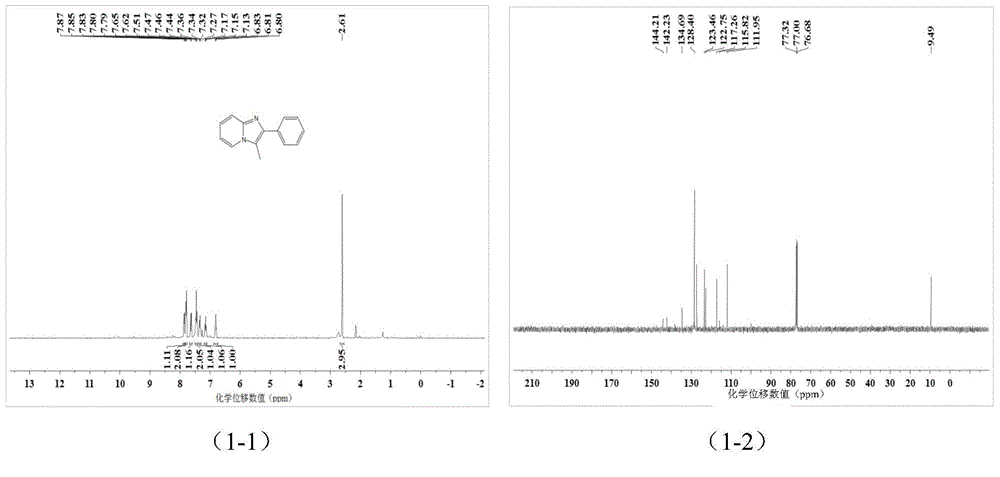
[0038] A kind of imidazopyridine compound is prepared by the following method:
[0039]Add 0.25 mmol 2-aminopyridine, 0.30 mmol 1-phenyl-2-propyne-1-acetate into a screw-top test tube with 2 ml of toluene, mix well and add 0.025 mmol cuprous chloride , stirred and reacted at 80°C for 5 hours, stopped heating and stirring, cooled to room temperature, removed the solvent by rotary evaporation under reduced pressure, and then separated and purified by column chromatography to obtain imidazopyridine compounds. The column chromatography eluent used was petroleum A mixed solvent of ether and ethyl acetate (the volume ratio of petroleum ether: ethyl acetate is 3:1), the yield is 85%.
Embodiment 2
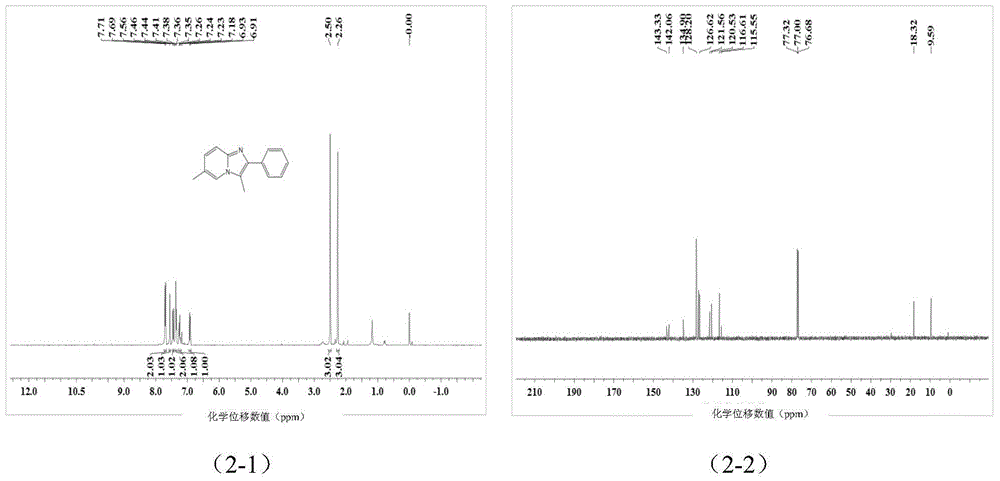
[0041] A kind of imidazopyridine compound is prepared by the following method:
[0042] Add 0.25 mmol 2-aminopyridine and 0.35 mmol 1-phenyl-2-propyne-1-acetate into a screw-top test tube with 2 ml of toluene, mix well and add 0.030 mmol cuprous chloride , stirred and reacted at 100°C for 6 hours, stopped heating and stirring, cooled to room temperature, removed the solvent by rotary evaporation under reduced pressure, and then separated and purified by column chromatography to obtain imidazopyridine compounds. The column chromatography eluent used was petroleum A mixed solvent of ether and ethyl acetate (the volume ratio of petroleum ether: ethyl acetate is 1:1), the yield is 80%.
Embodiment 3
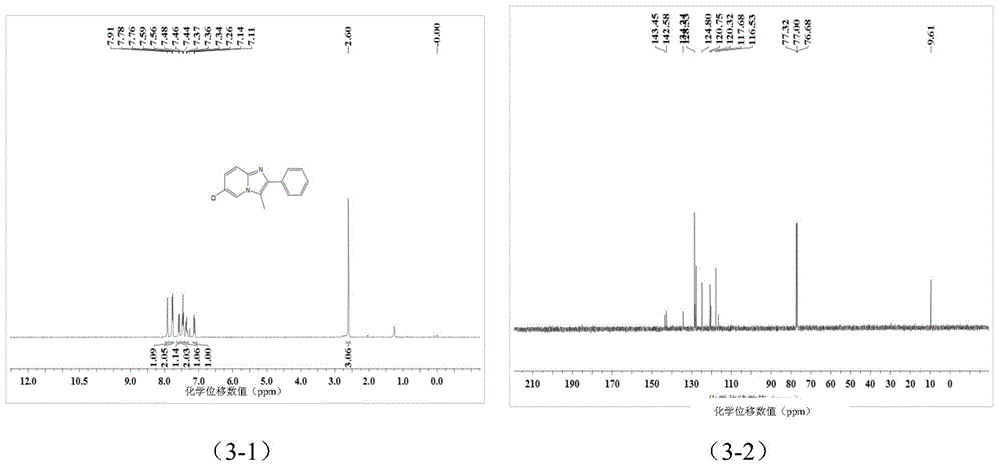
[0044] A kind of imidazopyridine compound is prepared by the following method:
[0045] Add 0.25 mmol 2-aminopyridine and 0.40 mmol 1-phenyl-2-propyne-1-acetate into a screw-top test tube with 2 ml of toluene, mix well and add 0.040 mmol cuprous chloride , stirred and reacted at 150°C for 3 hours, stopped heating and stirring, cooled to room temperature, removed the solvent by rotary evaporation under reduced pressure, and then separated and purified by column chromatography to obtain imidazopyridine compounds. The column chromatography eluent used was petroleum Mixed solvent of ether and ethyl acetate (the volume ratio of petroleum ether: ethyl acetate is 5:1), the yield is 83%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com