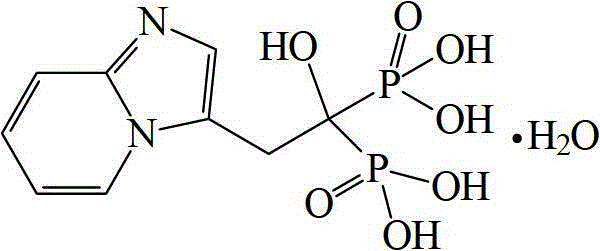
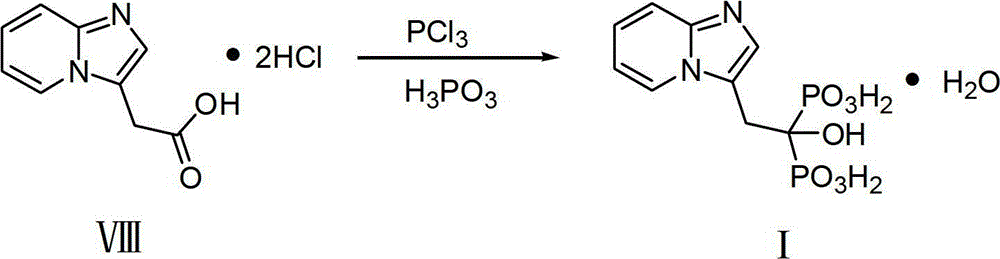
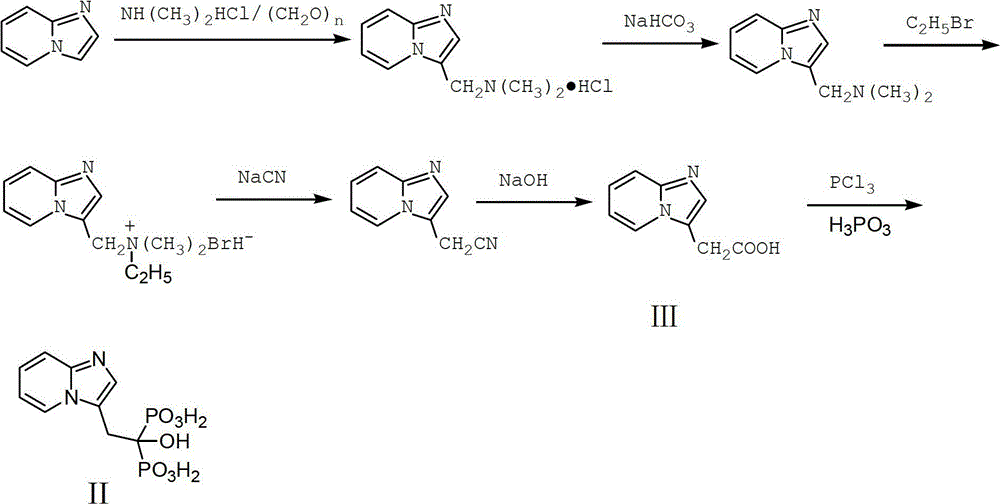
Preparation method of Minodronic acid hydrate
A technology of minodronic acid and hydrate, which is applied in the field of medicine and chemical industry, can solve the problems that are not suitable for large-scale industrial production, difficult to remove by-products, and low reaction yield, and achieve easy industrial production, low cost, and simple post-treatment Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0068] 1. Preparation of Compound (Ⅵ)
[0069] Add 165.0g (1.0mol) of ethyl 4-chloroacetoacetate to 112ml ethylene glycol (2.0mol) and mix well, add 1.375g (0.5%) of activated carbon-loaded phosphotungstic acid, add 1000ml cyclohexane, and heat to reflux. After reacting for 2 hours, filtering, and distilling off the solvent under reduced pressure, 181.4 g of oil was obtained, with a yield of 86.8%, namely compound (VI) (2-chloromethyl-[1,3]-dioxolan-2-yl) ethyl acetate.
[0070] 2. Preparation of Compound (Ⅳ)
[0071] Dissolve 180.0g of compound (Ⅵ) 2-chloromethyl-[1,3]-dioxolan-2-yl and 120.0g 2-aminopyridine in 1000ml of tetrahydrofuran, stir well, and slowly add 150.0g of triethylamine dropwise , heating to reflux reaction, TLC detection (ethyl acetate: methanol = 70:30) until the spot of compound (Ⅵ) disappears in the reaction solution, add 1000ml of water, then adjust the pH value of the reaction solution to 3.0 with 1N hydrochloric acid, and stir at room temperature fo...
Embodiment 2
[0079] 1. Preparation of Compound (Ⅵ)
[0080] Add 165.0g (1.0mol) of ethyl 4-chloroacetoacetate to 160ml of methanol (2.0mol) and mix well, add 1.465g (0.5%) of activated carbon-loaded phosphotungstic acid, add 1000ml of cyclohexane, heat to reflux, and react 2 hours, filtered, and the solvent was distilled off under reduced pressure to obtain 173.7 g of an oily substance with a yield of 82.3%, namely compound (Ⅵ) 4-chloro-3,3-dimethoxy-butyric acid ethyl ester.
[0081] 2. Preparation of Compound (Ⅳ)
[0082] Dissolve 170.0g of compound (VI) 4-chloro-3,3-dimethoxy-butyric acid ethyl ester and 113.0g of 2-aminopyridine in 1000ml of tetrahydrofuran, stir well, slowly add 142.0g of triethylamine dropwise, and heat to reflux Reaction, TLC detection (ethyl acetate: methanol = 70: 30) until the spot of compound (Ⅵ) disappears in the reaction solution, add 1000ml of water, then adjust the pH value of the reaction solution to 3.0 with 1N hydrochloric acid, stir the reaction at room...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com