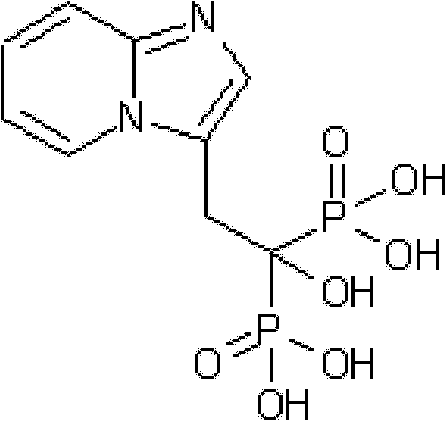
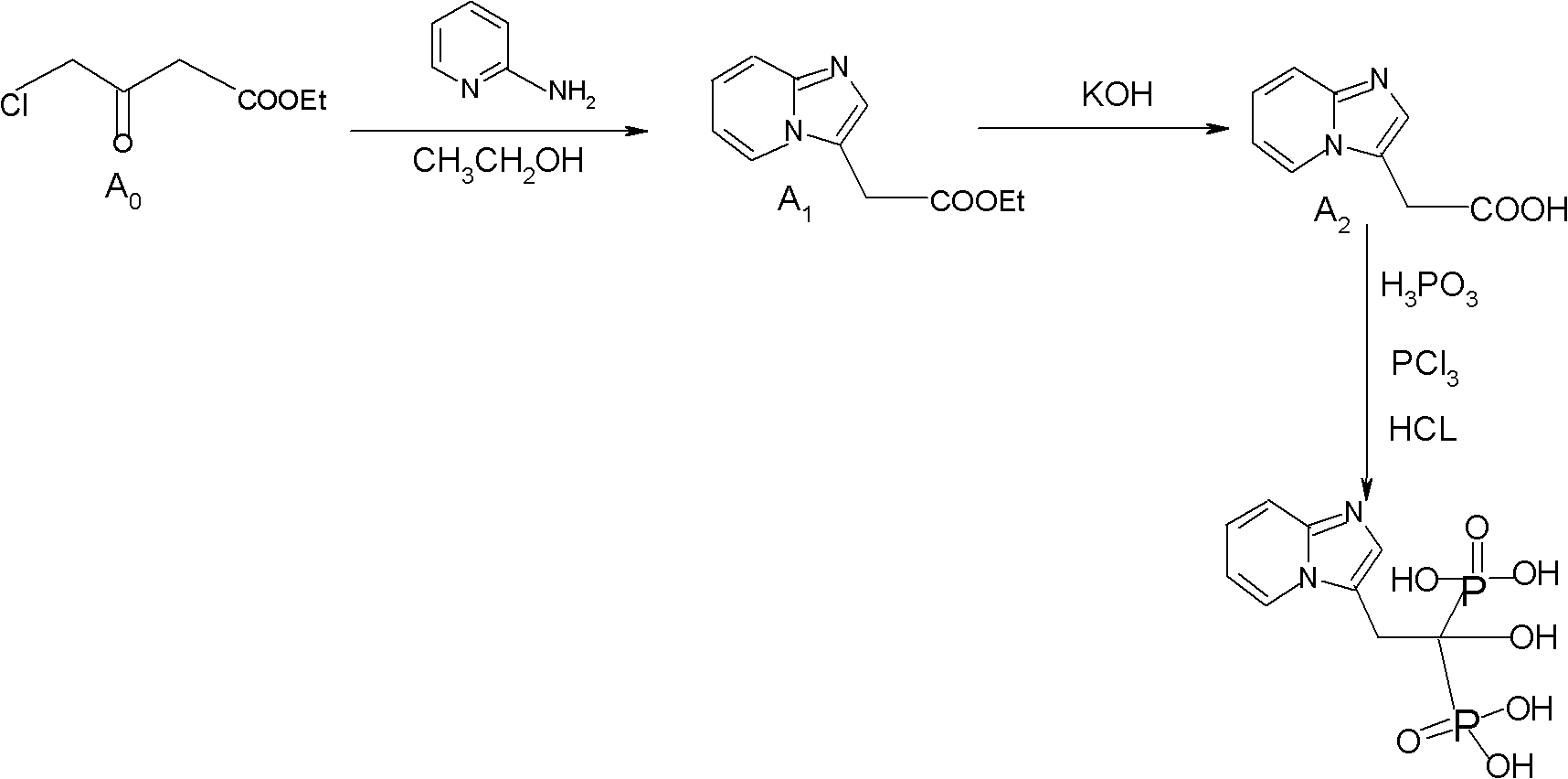
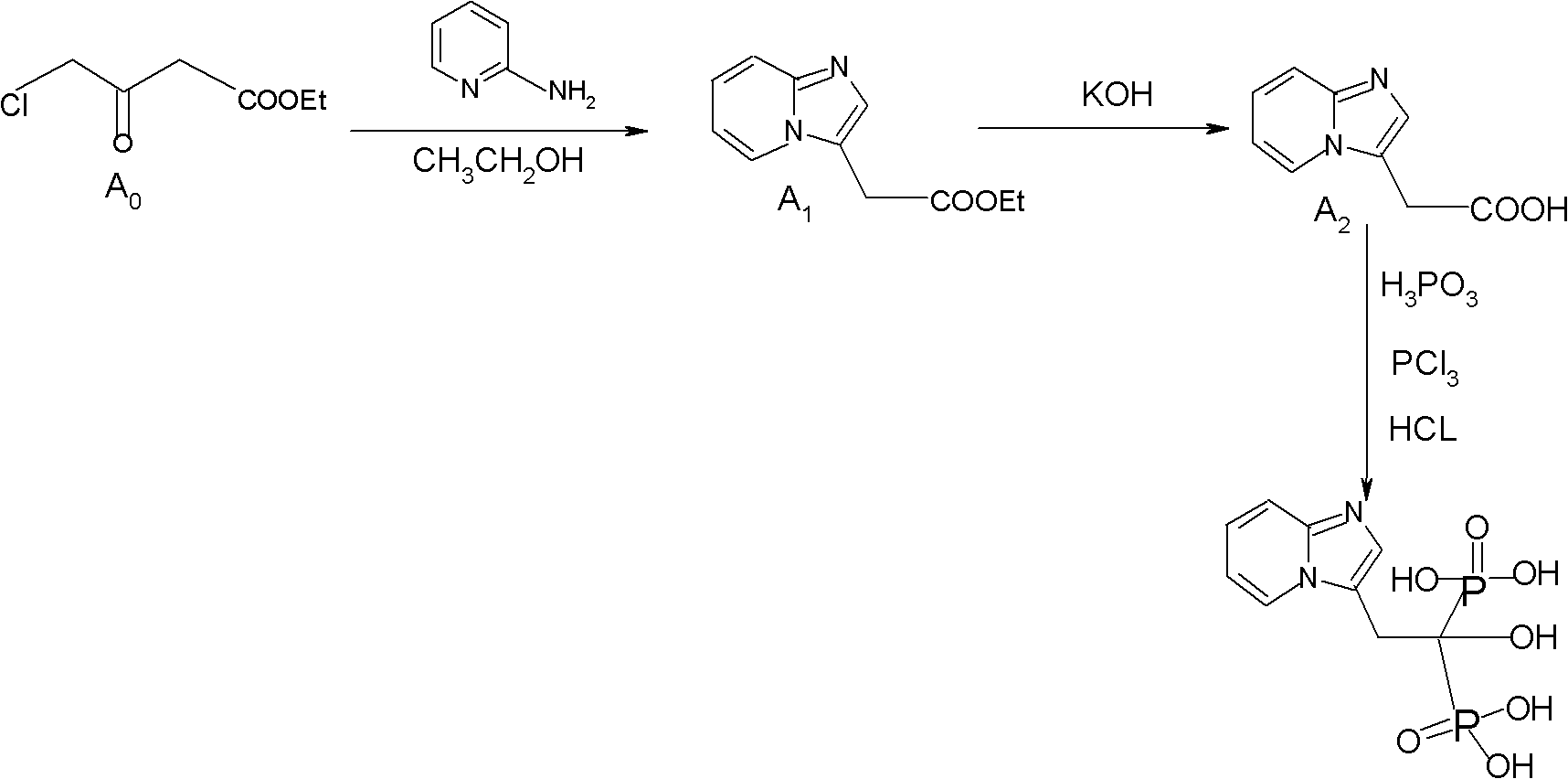
Synthesis method of minodronate midbody and synthesis of minodronate
A synthesis method and technology of minodronic acid are applied in the synthesis of minodronic acid intermediates and in the field of minodronic acid synthesis, which can solve the problems of high raw material cost, harsh conditions, and many by-products, and achieve stable product quality. , the effect of short reaction steps and reduced raw material cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] Embodiment 1, the preparation of A1
[0041] Add 7.21g of 2-aminopyridine and 48ml of ethyl acetate to a dry and anhydrous 250ml three-necked flask in sequence, stir well, protect with nitrogen, and dissolve all the solids. Control the temperature at 5--30°C and slowly add ethyl 4-chloroacetoacetate dropwise 6g / ethyl acetate 6ml mixed solution, dropwise completed in 15--30 minutes, keep warm at 30°C for 10 minutes, slowly raise the temperature to reflux for 2-3 hours, monitor the reaction solution by TLC until the spots of ethyl 4-chloroacetoacetate disappear (developing agent: ethyl acetate), after the reaction is completed, stop heating, reduce the temperature and concentrate to dryness under reduced pressure, and the concentrated residue is fully dissolved with 80 ml of purified water, and the aqueous layer is washed 8 times with 40 ml of n-hexane to remove impurities, and the n-hexane layer is discarded. The water layer was extracted 6 times with 50 ml of ethyl acet...
Embodiment 2
[0042] Embodiment 2, the preparation of A1
[0043] Add 7.21g of 2-aminopyridine and 48ml of absolute ethanol to a dry and anhydrous 250ml three-necked flask in sequence, stir well, protect with nitrogen, and dissolve all the solids. Control the temperature at 5--30°C and slowly add ethyl 4-chloroacetoacetate dropwise 6g / absolute ethanol 6ml mixed solution, add dropwise in 15--30 minutes, keep warm at 30°C for 10 minutes, slowly raise the temperature to reflux for 2-3 hours, monitor the reaction solution by TLC until the spots of ethyl 4-chloroacetoacetate disappear (developing agent: ethyl acetate), after the reaction is completed, stop heating, reduce the temperature and concentrate to dryness under reduced pressure, and the concentrated residue is fully dissolved with 80 ml of purified water, and the aqueous layer is washed 8 times with 40 ml of n-hexane to remove impurities, and the n-hexane layer is discarded. The water layer was extracted 6 times with 50 ml of ethyl acet...
Embodiment 3
[0044] Embodiment 3, the preparation of A1
[0045] Add 7.21g of 2-aminopyridine, 48ml of absolute ethanol, and 0.24g of potassium iodide to a dry and anhydrous 250ml three-necked flask in sequence, stir well, protect with nitrogen, and dissolve all the solids. Slowly add 4-chloropyridine at a temperature of 5-30°C. Ethyl acetoacetate 6g / absolute ethanol 6ml mixed solution, add dropwise in 15--30 minutes, keep warm at 30°C for 10 minutes, slowly raise the temperature to reflux for 3 hours, TLC monitors the reaction solution to 4-chloroacetoacetate ethyl Spots disappear (developing agent: ethyl acetate), after the reaction is complete, stop heating, reduce the temperature and concentrate to dryness under reduced pressure, and the concentrated residue is fully dissolved with 80ml of purified water, and the aqueous layer is washed 8 times with 40ml of n-hexane to remove impurities. The layer was discarded, the aqueous layer was extracted 6 times with 50 ml of ethyl acetate, the e...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com