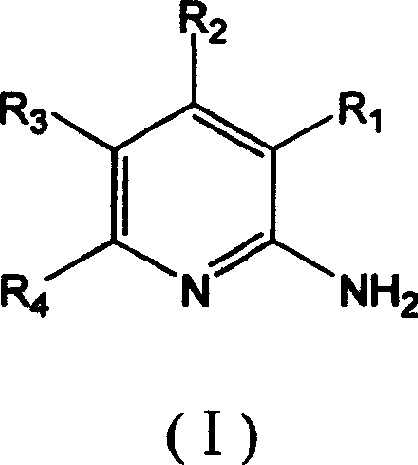
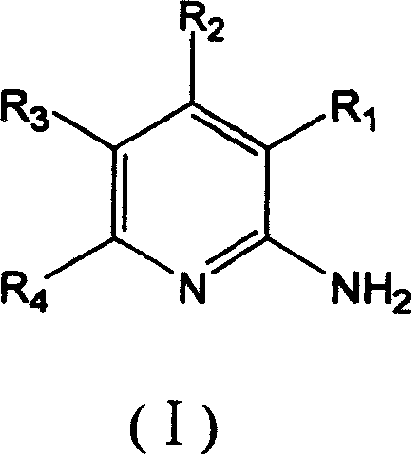
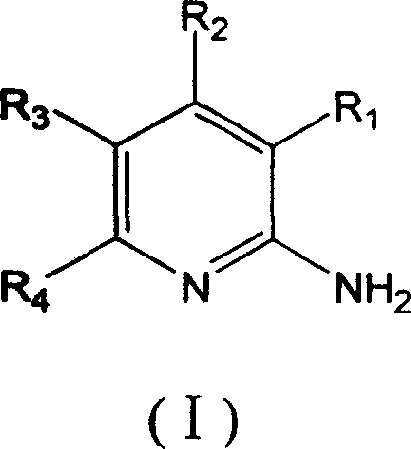
Method for preparing 2-aminopyridine and alkyl derivative
A technology for aminopyridine and derivatives, which is applied in the field of preparing 2-aminopyridine and its alkyl derivatives, can solve the problems of unsatisfactory product quality, high equipment requirements, low product yield and the like, and achieves easy operation and low equipment requirements. , the effect of simple reaction route
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] Example 1: 2-aminopyridine
[0025] 1) 2-cyanopyridine (20.8g, 0.2mol) was dropped into a mixed solvent of water (50mL) and acetone (50mL), and 10% H was added dropwise within 15 minutes under stirring 2 o 2 (123.5mL), heated up to 50°C, continued to stir for 2.5 hours, stopped the reaction, refrigerated, filtered with suction, and dried to obtain 23.4g of white solid 2-amidopyridine, which was directly used in the next reaction.
[0026] 2) Put the white solid obtained in the previous step into 12% NaOH aqueous solution (200mL), and add Br dropwise at 0°C 2 (10.5mL), keep the temperature at about 0°C, after the dropwise addition is complete, raise the temperature to 60°C for 0.5 hour reaction, and stop the reaction. The reaction solution was cooled, extracted twice with ethyl acetate (50 mL), the organic phase was washed twice with water (50 mL), dried, and concentrated to give a red liquid, which was distilled under reduced pressure (20 mmHg, 82-83° C.) to give 16.6...
Embodiment 2
[0027] Example 2: 2-Aminopyridine
[0028] The acetone was replaced with ethanol (50ml), and the rest was the same as in Example 1 to obtain 16.2g of white solid with a yield of 86.1% and a melting point of 60-61°C.
Embodiment 3
[0029] Example 3: 2-amino-3-picoline
[0030] The process was as in Example 1, using 2-cyano-3-picoline as raw material. The crude product was distilled under reduced pressure (20mmHg, 107-108.5°C) to obtain a white solid with a yield of 87.4% and a melting point of 33.5°C.
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com