In situ thermosensitive gel medicine delivery system for immunochemotherapy combination therapy
A temperature-sensitive gel and combined therapy technology, applied in drug delivery, drug combination, liquid delivery, etc., can solve systemic side effects, barriers and other problems, achieve the effects of reducing systemic side effects, overcoming systemic toxicity, and improving curative effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] The following specific examples are further descriptions of the present invention, but the following are only used to illustrate the present invention rather than limit the scope of the present invention. Embodiment 1: Preparation of PLGA-PEG-PLGA temperature-sensitive material
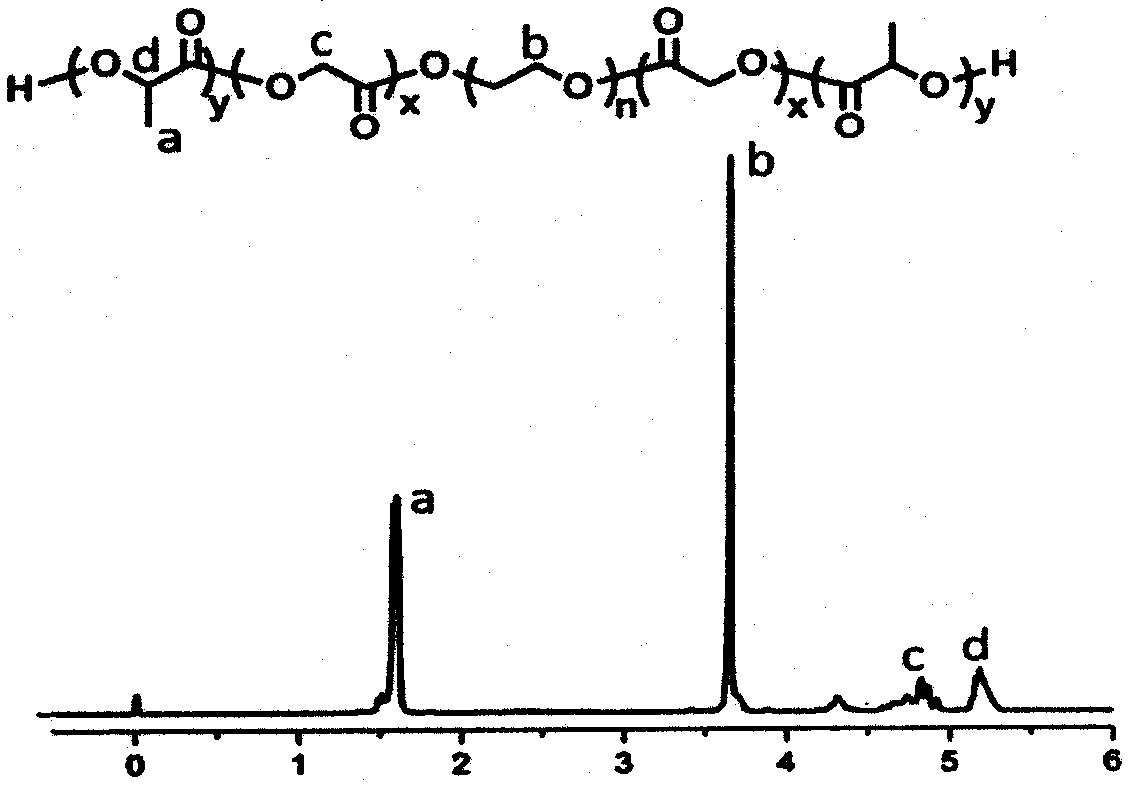
[0043] 20.06g of PEG with Mn~1000Da was dehydrated under vacuum at 120°C for 4h. After the temperature dropped to 80°C, 4.21gGA and 37.18g LA were added and dehydrated for 30min under the protection of argon. Then 0.15% stannous octoate (w / w) of LA and GA feedstock was added as a catalyst. After reacting at 150°C for 12 hours, the product was washed with hot water at 80°C three times to purify the product.
[0044] for PLGA-PEG-PLGA 1 H-NMR analysis (attached figure 1 ), the ratio of lactide (LA) to glycolide (GA) is 1.7 / 1, the molecular weight is 1250-1000-1250, and the gel permeation chromatography (GPC) obtains Mw / Mn=1.4.
Embodiment 2
[0045] Embodiment 2: Preparation of peptide dendrimers:
[0046] 1. H-Lys-OMe (699.4mg, 3mmol), Boc-L-Lys(Boc)-OH (2.18g, 6.3mmol), hexafluorophosphate (HBTU, 2.39g, 6.3mmol) were mixed under nitrogen protection and 1-hydroxybenzotriazole (HOBt, 851.1 mg, 6.3 mmol) were dissolved in anhydrous dimethylformamide (DMF, 50 mL). Diisopropylethylamine (DIPEA, 1.63 g, 12.6 mmol) was added and stirred in an ice bath for 5 minutes, then tris(2-aminoethyl)amine (220 mg, 1.5 mmol) was added. Stir in an ice bath under nitrogen for 30 minutes, then at room temperature for 8 hours. After the reaction was completed, the solvent was removed, the residue was dissolved in ether (150 mL), and saturated NaHCO 3 Aqueous solution, NaHSO 4 Aqueous solution (1M), saturated NaHCO 3 Aqueous solution and water wash. use Na 2 SO 4 The solution was dried and the solvent was removed by rotary evaporation to give a white solid.
[0047] 2. The compound obtained in the previous step (2.26 g, 2 mmol) ...
Embodiment 3
[0050] Example 3: Preparation of drug-loaded peptide dendrimers
[0051] Dissolve 10 mg of peptide dendrimers in 1.5 ml of water, 15 mol equivalent DOX·HCL in 300 μL of methanol, add 5 μL of triethylamine to alkalinize, then add the DOX solution into the peptide dendrimer solution, and stir overnight Afterwards, the mixed solution was centrifuged (8000 rpm for 5 min), and the supernatant was freeze-dried to obtain the drug-loaded peptide dendrimers.
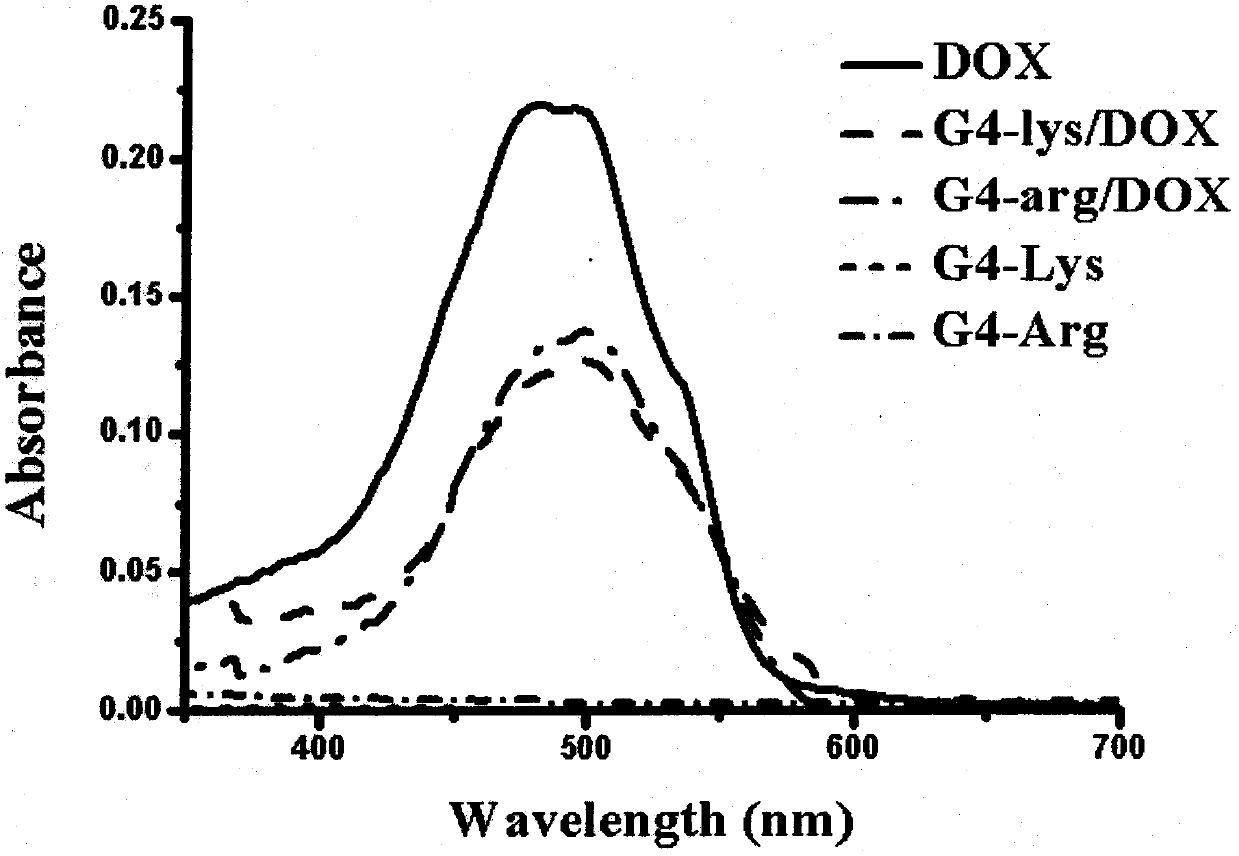
[0052] The drug loading capacity of the peptide dendrimers was determined to be 17 μg / mg by ultraviolet light. And the ultraviolet absorption spectrum scanning of the drug-loaded peptide dendrimers was carried out (attached Figure 4 ), the maximum absorption peak of the drug-loaded peptide dendrimers has a slight red shift.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Volume | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com