Pyridylurea biquaternary ammonium salt as well as preparation method and application thereof
A technology of double quaternary ammonium salt and pyridine urea, applied in the field of pesticides, can solve the problems of high production cost, complex synthesis process, limited use and promotion, etc., and achieve the effect of solving poor water solubility, good water solubility, and promoting plant cell division
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
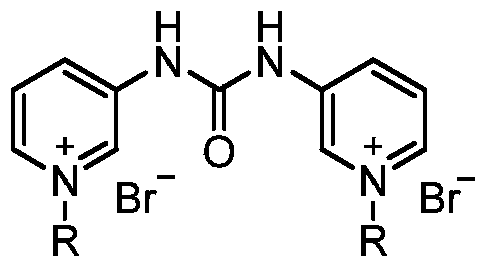
[0020] Preparation of 1,3-bis(N-octyl-3-pyridyl)urea bromide diquaternary ammonium salt
[0021] (1) Preparation of 1,3-di(3-pyridyl)urea: In a reaction flask, add 4.51g (48mmol) 3-aminopyridine, 3.53mL (48mmol) triethylamine and 80mL dichloromethane, start stirring , cooled to 0°C with an ice-water bath, dropwise added 2.85g (9.6mmol) of triphosgene in dichloromethane solution, controlled the rate of addition to keep the temperature of the reaction system below 5°C, the dropwise addition was completed in about half an hour, and then heated to reflux for reaction After 5 hours, cool, filter, wash with 10% aqueous sodium carbonate solution, wash with water, and dry to obtain 4.16 g of 1,3-bis(3-pyridyl)urea with a yield of 81%. 1 HNMR (300MHz, δppm, DMSO-d 6 ):8.97(s,2H),8.59(s,2H),8.18(d,2H),7.91(d,2H),7.30(t,2H).
[0022] (2) In the reaction flask, add 3.00g (14mmol) 1,3-bis(3-pyridyl)urea and 20mL (114.8mmol) n-octane bromide, heat to 120°C for 3 hours, cool, filter, Wash...
Embodiment 2
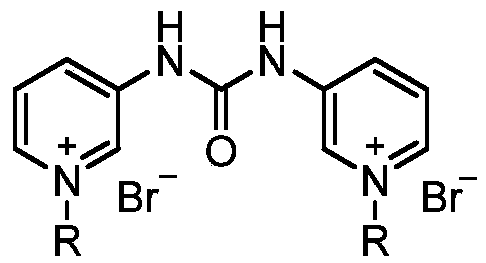
[0024] Preparation of 1,3-bis(N-decyl-3-pyridyl)urea bromide diquaternary ammonium salt
[0025] (1) The preparation of 1,3-bis(3-pyridyl)urea is the same as in Example 1;
[0026] (2) In the reaction flask, add 3.00g (14mmol) 1,3-bis(3-pyridyl)urea and 20mL (96.7mmol) brominated n-decane, heat to 110°C for 4 hours, cool, filter, Wash with acetone and dry to obtain 8.37 g of 1,3-di(N-n-decyl-3-pyridyl)urea diquaternary ammonium bromide, with a yield of 91.2% and a melting point of 252-253°C. 1 HNMR (300MHz, δppm, CDCl 3 ):10.99(s,2H),9.27(m,4H),8.45(m,2H),8.04(d,2H),4.80(t,4H),2.04(m,4H),1.44-1.21(m, 28H),0.83(m,6H).
Embodiment 3
[0028] Preparation of 1,3-bis(N-n-dodecyl-3-pyridyl)urea bromide diquaternary ammonium salt
[0029] (1) The preparation of 1,3-bis(3-pyridyl)urea is the same as in Example 1;
[0030] (2) In the reaction flask, add 3.00g (14mmol) 1,3-bis(3-pyridyl)urea and 20mL (83.5mmol) n-dodecane bromide, heat to 100°C for 5 hours, cool and filter , washed with acetone, and dried to obtain 9.00 g of 1,3-bis(N-n-dodecyl-3-pyridyl)urea diquaternary ammonium bromide, yield 90.3%, melting point: 254-255°C (dec) . 1 HNMR (300MHz, δppm, CDCl 3 ):11.04(s,2H),9.28(d,2H),9.21(s,2H),8.48(d,2H),8.09(m,2H),4.85(t,4H),2.08-1.24(m, 40H), 0.88(t,6H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com