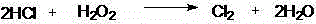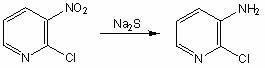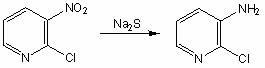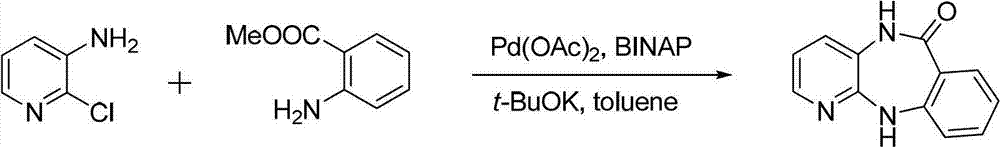Patents
Literature
67 results about "3-Aminopyridine" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
3-Aminopyridine is an aminopyridine. It is a colorless solid.
Preparation method for 2,3-dichloropyridine
ActiveCN103570609AReduce generationSave energyOrganic chemistrySandmeyer reactionBULK ACTIVE INGREDIENT
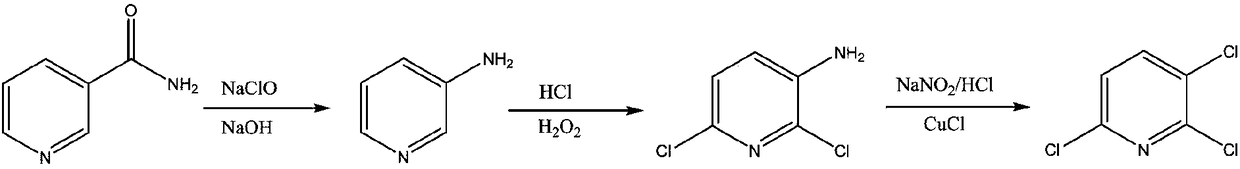
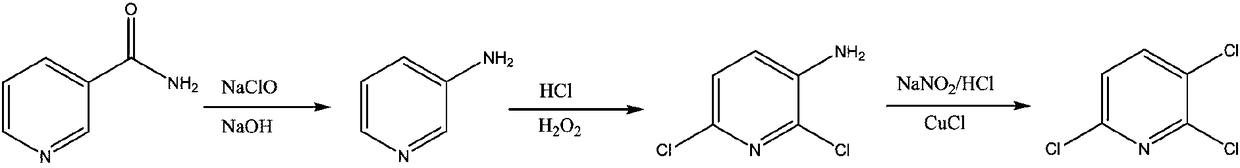
The invention discloses a preparation method for 2,3-dichloropyridine. The preparation method creatively comprises the steps of reacting nicotinamide serving as an active ingredient with sodium hypochloride to prepare 3-aminopyridine, distilling to remove water, extracting with dichloromethane to recover 3-aminopyridine, wherein the yield of the 3-aminopyridine is more than or equal to 93%; reacting the 3-aminopyridine hydrochloric acid solution with hydrogen peroxide to obtain 2-chlorino-3-aminopyridine hydrochloric acid solution, wherein the yield of the 2-chlorino-3-aminopyridine is more than or equal to 85%; then performing diazotization and sandmeyer reaction to obtain 2,3-dichloropyridine. The preparation method is less in side reaction, an intermediate product is not required for refining, and the product is high in content and yield, simple in technology and easy to operate.
Owner:NANTONG TENDENCI CHEM
Method for preparing 2,3-dichloropyridine
The invention discloses a method for preparing 2,3-dichloropyridine with a simple process and a high yield rate. The method is as follows: in a concentrated hydrochloric acid, 3-aminopyridine is used as a starting material, Fe<2> or Fe <3> is used as a chlorination catalyst, and a mixture of hydrogen peroxide and hydrochloric acid or chlorine is used as a chlorating agent to conduct a chlorination reaction to chloridize the 3-aminopyridine. The reaction mixtures without being separated are added with Cu<+> or Cu<2+> which serves as a catalyst for diazotization / chlorination reaction and with an aqueous solution sodium nitrite to conduct diazotization / chlorination reaction to prepare 2,3-dichloropyridine by a one-pot process. The 2,3-dichloropyridine is isolated and purified by a normal method with a purity more than 99.2 percent. According to a 3-aminopyridine based calculation, the molar yield rate of the 2,3-dichloropyridine is more than 74.1 percent.
Owner:CANGZHOU LINGANG YANUO CHEM CO LTD
2,3-dichloropyridine synthesis method
InactiveCN1807414ASuppress generationLow reaction temperatureOrganic chemistry3-AminopyridineCopper chloride
A Synthesis of 2, 3-dichloro-pyridine, including steps as follows: 1)liquating the raw material of 3-aminopyridine in decuple-twelvefold mass thick alcaine at the condition of mixing, cooling to 4-5 deg C, adding equal mol mass hydroperoxide as raw material, controlling reaction temperature between 6-8deg Cand mixing for 1-2h at the same temperature after adding the things; 2) cooling the solution below 0deg C, adding equal mol mass erinitrit solution as raw material and then preserving heat for 0. 5-1h; 3) controlling the reaction temperature below 0deg C, adding miscible liquids of 0. 15 times mol mass copper chloride as the raw material and duple mass as thick alcaine, then preserving heat for a half hour at least; 4) Returning reactant to ambient temperature and doing extraction by dichloromethane; 5) vacuum distilling the tobacco extract to dry will get product. The intermediate products of the invention are never purified and moved, all the procedures can be done in the same reaction pot and the three step reaction only uses alcaine which contains reactant as solution.
Owner:南京广通医药化工有限责任公司
Antiviral agents and methods of treating viral infections
InactiveUS20020188011A1Enhance the beneficial effectEasy to modifyBiocideCarbohydrate active ingredients4-Methylpyridine3-Aminopyridine
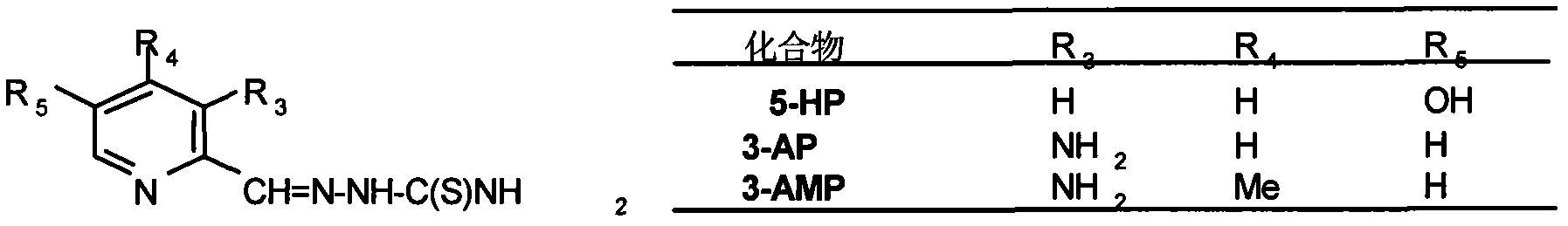
The present invention relates to methods of treating viral or fungal infections using 3-aminopyridine-2-carboxyaldehyde thiosemicarbazone (3-AP) and 3-amino-4-methylpyridine-2-carboxaldehyde thiosemicarbazone (3-AMP) and its prodrug forms and to pharmaceutical compositions comprising these compounds.
Owner:VION PHARMA INC +1
2,3-dichloropyridine synthesis method
InactiveCN100357272CSuppress generationLow reaction temperatureOrganic chemistry3-AminopyridineCopper chloride
A Synthesis of 2, 3-dichloro-pyridine, including steps as follows: 1)liquating the raw material of 3-aminopyridine in decuple-twelvefold mass thick alcaine at the condition of mixing, cooling to 4-5 deg C, adding equal mol mass hydroperoxide as raw material, controlling reaction temperature between 6-8deg Cand mixing for 1-2h at the same temperature after adding the things; 2) cooling the solution below 0deg C, adding equal mol mass erinitrit solution as raw material and then preserving heat for 0. 5-1h; 3) controlling the reaction temperature below 0deg C, adding miscible liquids of 0. 15 times mol mass copper chloride as the raw material and duple mass as thick alcaine, then preserving heat for a half hour at least; 4) Returning reactant to ambient temperature and doing extraction by dichloromethane; 5) vacuum distilling the tobacco extract to dry will get product. The intermediate products of the invention are never purified and moved, all the procedures can be done in the same reaction pot and the three step reaction only uses alcaine which contains reactant as solution.
Owner:南京广通医药化工有限责任公司
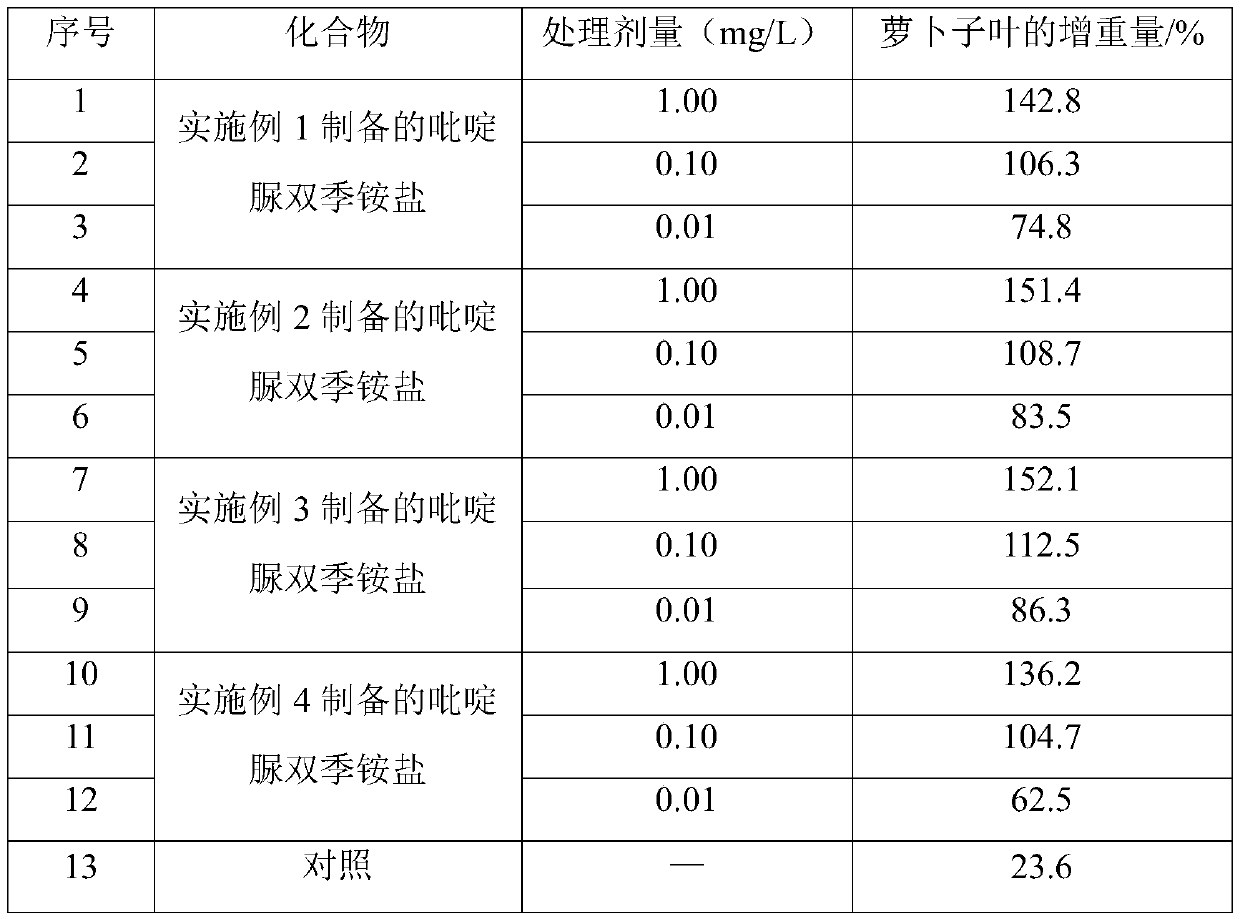
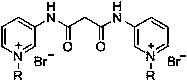
Pyridylurea biquaternary ammonium salt as well as preparation method and application thereof
ActiveCN105503711APromote divisionGood water solubilityPlant growth regulatorsBiocideSolubilitySolvent free
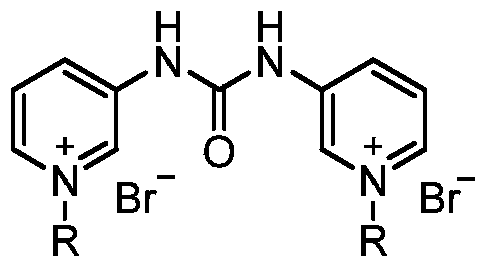
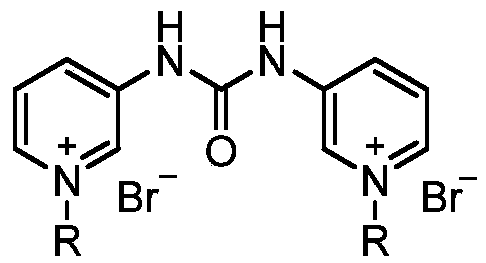
The invention relates to a pyridylurea biquaternary ammonium salt as well as a preparation method and application thereof. The preparation method of the pyridylurea biquaternary ammonium salt comprises the following steps: using 3-aminopyridine as a staring material, conducting reaction on 3-aminopyridine and triphosgene to obtain 1,3-bi(3-pyridyl)urea, and conducting reaction on 1,3-bi(3-pyridyl)urea and alkyl bromide under the solvent-free condition to prepare the pyridylurea biquaternary ammonium salt. According to the method, a solvent-free method is adopted to prepare the pyridylurea biquaternary ammonium salt, so that the environment can be protected, and the yield is relatively high. The provided pyridylurea biquaternary ammonium salt can be used as a plant growth regulator to promote plant cell division, and the problem that the traditional phenylurea plant growth regulator is poor in water solubility is solved.
Owner:SHANXI UNIV
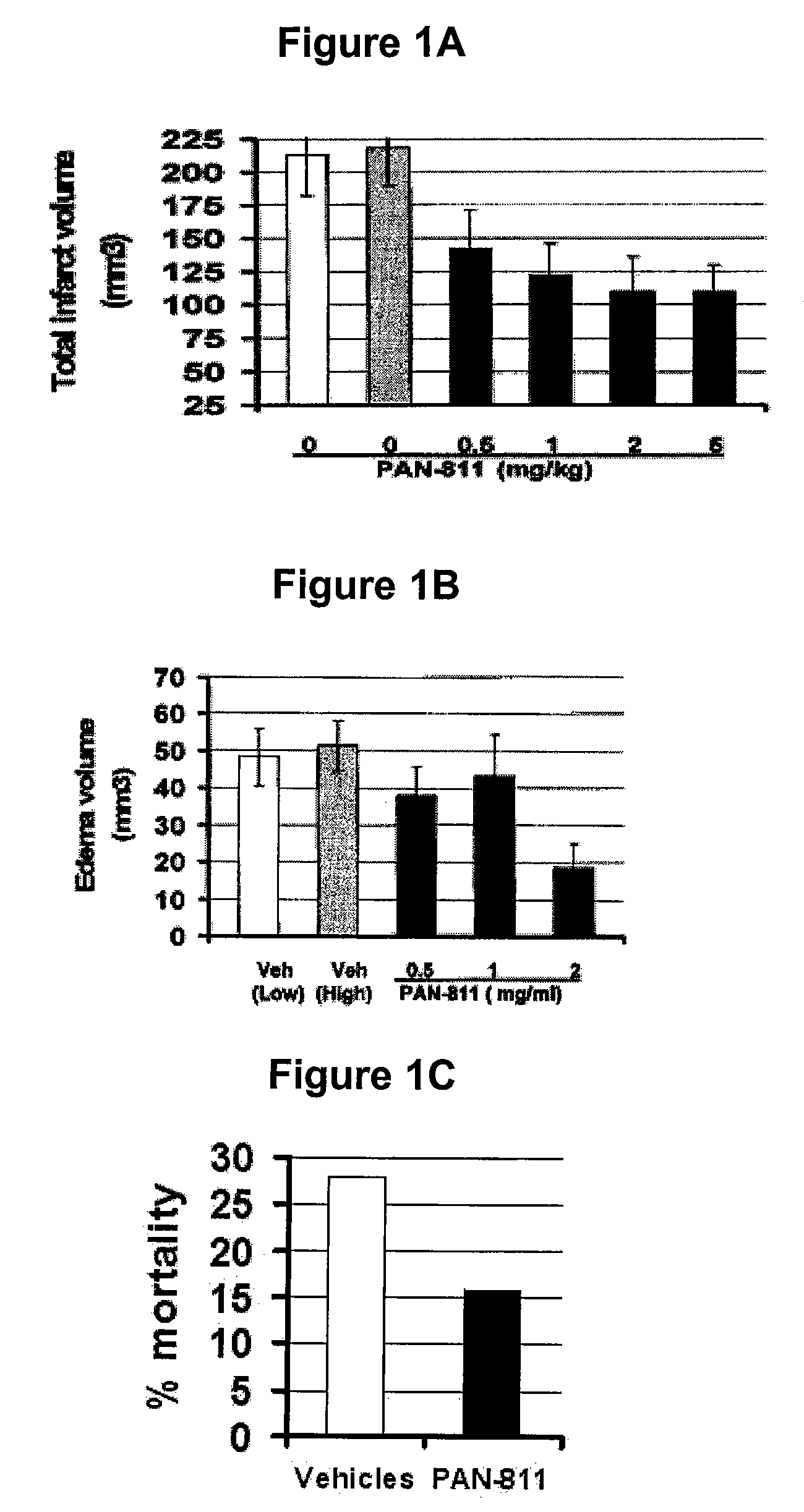
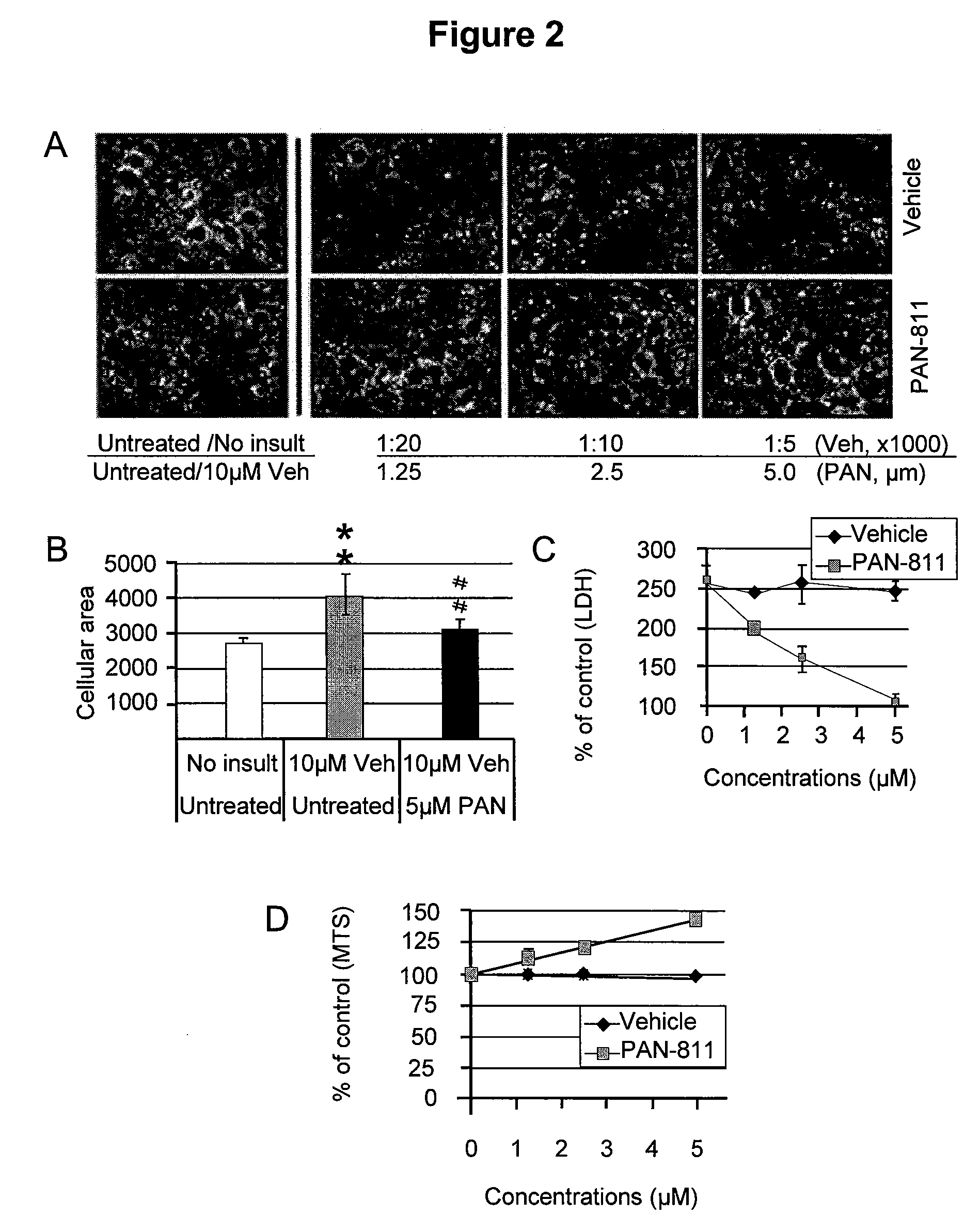
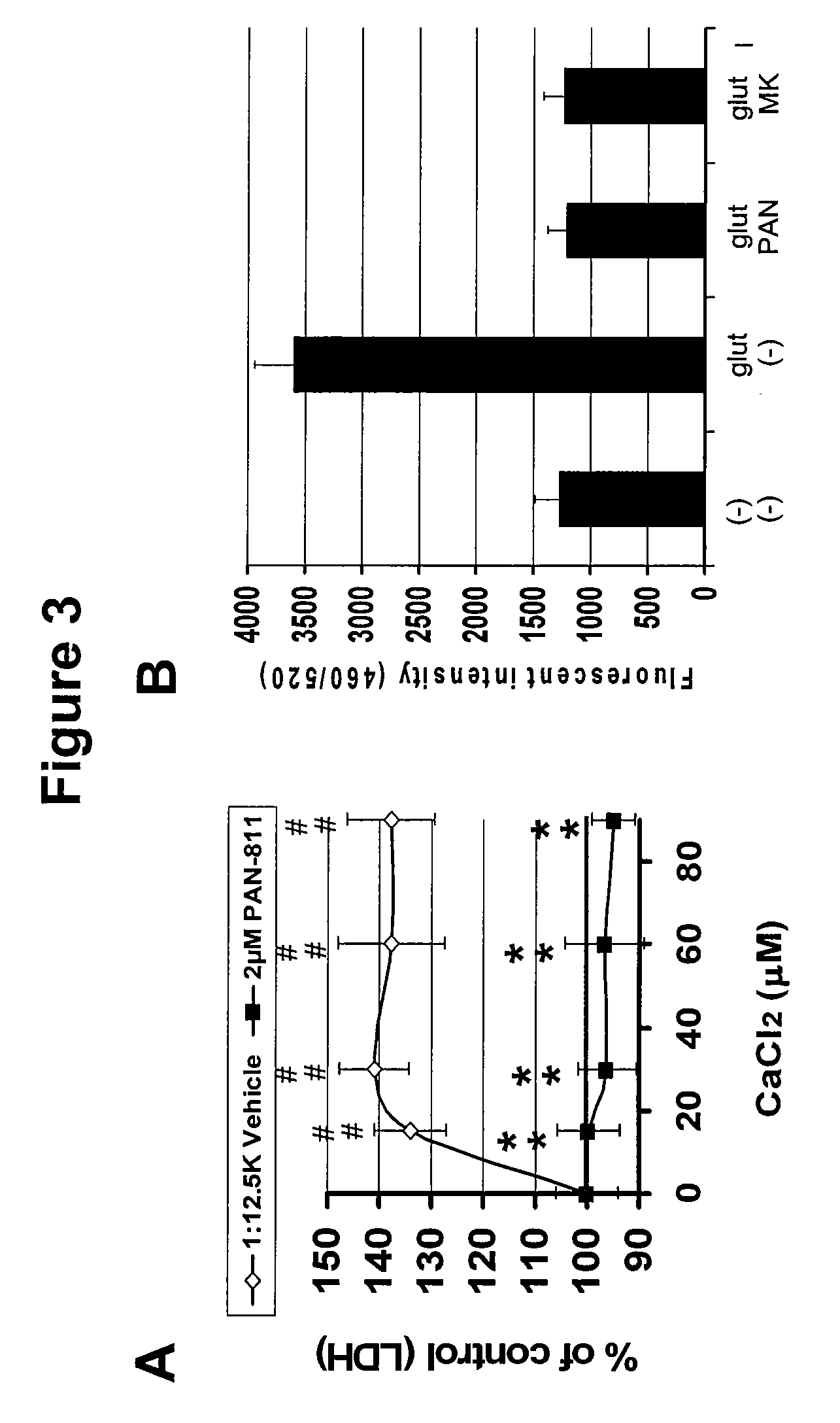
Methods for the treatment of brain edema
InactiveUS20090286799A1Reduced infarct volumeReduce mortalityBiocideAnimal repellantsMortality rateCalcium influx
The present invention is based on the discoveries that PAN-811 (1) reduces infarct volume, suppresses brain edema and decreases mortality associated with ischemia; (2) blocks veratridine-induced swelling and neuronal cell death; (3) chelates free calcium and inhibits MMP-9 activity; and (4) blocks calcium-induced neuronal cell death and suppresses glutamate-induced calcium influx into neuronal cells. More particularly, the present invention relates to methods for treating, ameliorating or preventing vasogenic and / or cytotoxic brain edema, by administering to a subject in need thereof certain thiosemicarbazone compounds or pharmaceutically acceptable salts thereof. An example of such a thiosemicarbazone is 3-aminopyridine-2-carboxaldehyde thiosemicarbazone (PAN-811).
Owner:PANACEA PHARMA
Use of potassium channel blockers to treat cerebral palsy
InactiveUS20130030025A1Signs improvedSymptoms improvedBiocideNervous disorder3-AminopyridinePotassium
Disclosed herein is the use of aminopyridines, such as 3-aminopyridine, 4-aminopyridine or 3,4-diaminopyridine, in the management and treatment of cerebral palsy patients of all ages.
Owner:ACORDA THERAPEUTICS INC
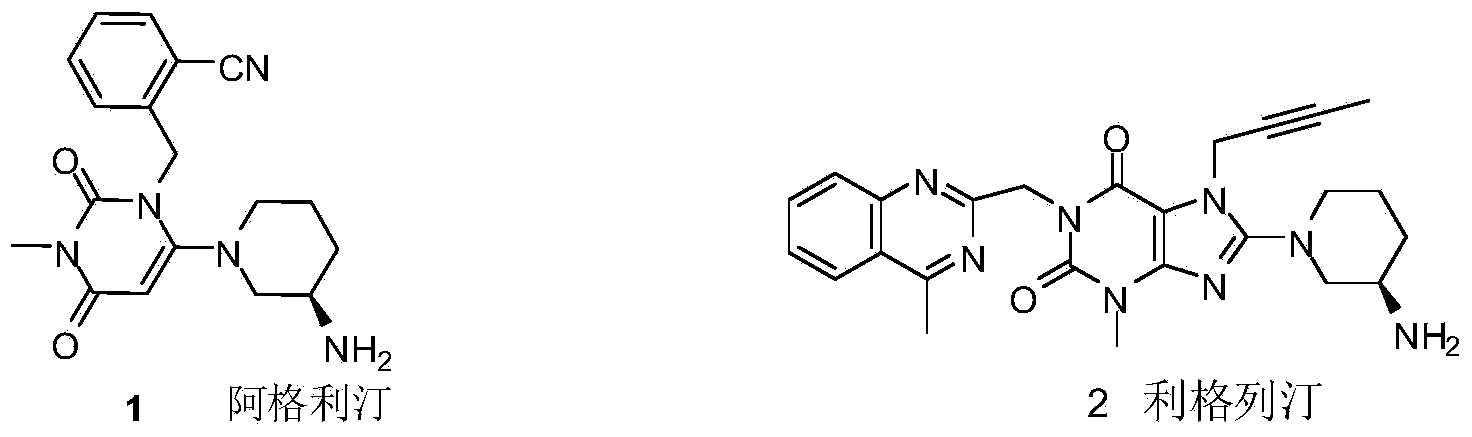
Compound I and (R)-3-aminopiperidine hydrochloride II, preparation method and application in Linagliptin synthesis
ActiveCN104387315AHigh optical purityHigh process reproducibilityOptically-active compound separationOrganic racemisationDipeptidyl peptidase3-Aminopyridine
The invention discloses a preparation method of 3-aminopiperidine and its derivative with optical activity and an application of the compound and its derivative in synthesis of a dipeptidyl peptidase-IV inhibitor Linagliptin. According to the preparation method, 3-aminopyridine is used as a raw material to prepare a compound I by 3- amino protection, catalytic hydrogenation reduction of pyridine ring and chiral reagent resolution, and deprotection is then carried out to obtain (R)-3-aminopiperidine hydrochloride II. In the application, a compound III prepared from the compound I is an important intermediate for synthesis of Linagliptin, and (R)-3-aminopiperidine hydrochloride II also can directly be used for synthesis of high-purity Linagliptin. The raw material used in the invention is low-cost and is easily-available in the market; each step is simple to operate; requirements on equipment are low; safety is high; and the preparation method is easy for industrial production.
Owner:2Y CHEM
Benzaldehyde Schiff base type aminopyridine acetylated starch and preparation method thereof
ActiveCN105199006AHigh reactivityImprove biological activityAntibacterial agentsDisinfectantsSolubilityBenzaldehyde
The invention relates to the daily chemical field and the pharmaceutical industry, in particular to benzaldehyde Schiff base type aminopyridine acetylated starch and preparation thereof. The structural formula of the benzaldehyde Schiff base type aminopyridine acetylated starch is shown as the formula (1). A preparation method comprises the following steps: firstly, starch reacts with chloroacetyl chloride, then various types of benzaldehyde react with 3-aminopyridine, Schiff bases are prepared, chloroacetyl starch reacts with the prepared Schiff bases finally, and the benzaldehyde Schiff base type aminopyridine acetylated starch shown as the formula (1) is obtained through purification, wherein the molar weight of chloroacetyl chloride is 1-2 times that of the starch, and the molar weight of various types of the Schiff bases is 2-3 times that of the chloroacetyl starch. The reaction is simple, convenient and efficient, popularization is easy, and required equipment and raw materials are easy to obtain. Research proves that the derivative has good water solubility and bacteriostatic activity, the biological activity of the starch is enhanced, the application range of the starch is enlarged, and the starch and the preparation method can be widely applied to the fields of daily chemical and pharmaceuticals.
Owner:YANTAI INST OF COASTAL ZONE RES CHINESE ACAD OF SCI
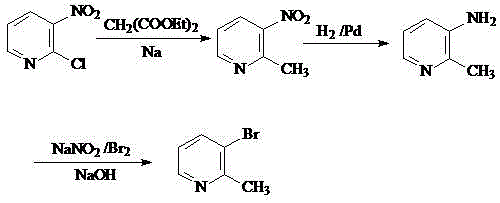
Preparation method of 2-methyl-3-bromopyridine
The invention belongs to the field of organic synthesis and particularly relates to a preparation method of 2-methyl-3-bromopyridine. The preparation method includes the following steps that 1, diethyl malonate reacts with alkali metal to generate salt, then a methylbenzene solution of 2-chlorine-3-nitropyridine is added dropwise to be conducted a condensation reaction, and the 2-methyl-3-nitropyridine is obtained through decarboxylation under an acidic condition; 2, under the catalysis of Pd / C, methanol serves as a solvent, a hydrogenation reduction and suction filtration are conducted on the 2-methyl-3-nitropyridine, filtered liquid is condensed, and 2-methyl-3-aminopyridine is obtained; 3, the 2-methyl-3-aminopyridine reacts with acid to generate salt, the cooling is conducted to enable the temperature to be in -10 DEG C-0 DEG C, bromine is added dropwise, after the addition, then a sodium nitrite solution is added dropwise, after addition, pH of the solution is adjusted to be alkaline, then extracting, drying and concentration are conducted, and the 2-methyl-3-bromopyridine is obtained. The preparation method of the 2-methyl-3-bromopyridine has the advantages that the reaction condition is moderate, the operation is easy, the post-processing is simple, enlarged production is easy, and the preparation method is very suitable for industrial production; the catalysis effect is good, and the reaction yield ratio is high; the price of the raw material is low, and the production cost is low.
Owner:洪帅金
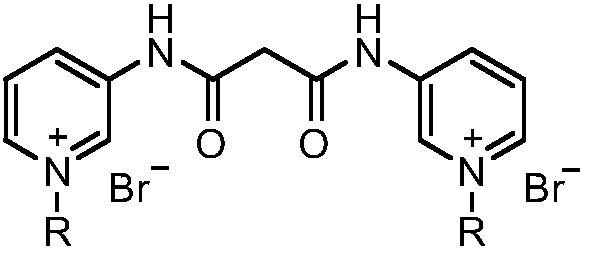
Pyridine bis-quaternary ammonium salt surfactant and preparation method and application thereof
PendingCN109096183ARaw materials are easy to getEasy to makeBiocideOrganic chemistryEscherichia coliAmpicillin
The invention discloses a pyridine bis-quaternary ammonium salt surfactant and a preparation method and an application thereof. The preparation method of the pyridine bis-quaternary ammonium salt surfactant includes utilizing 3-aminopyridine as a starting raw material to react with diethyl malonate to obtain N,N'-di(3-pyridyl) malonamide and then reacting with brominated alkane under a solvent-free condition to obtain the pyridine bis-quaternary ammonium salt surfactant. The pyridine bis-quaternary ammonium salt surfactant not only has good surface activity with the critical micelle concentration as low as 1.69*10^5 mol L-1, but also has excellent antibacterial property with the minimum semi-inhibitory concentration of escherichia coli as 2.699-6.538Mug / mL, and the antibacterial effect isbetter than that of ampicillin.
Owner:SHANXI UNIV
Preparation method for high purity 2-chlorine-3-aminopyridine hydrochloride
The invention discloses a preparation method for high purity 2-chlorine-3-aminopyridine hydrochloride, which comprises choosing 3-aminopyridine as a raw material to be chloridized to obtain 2-chlorine-3-aminopyridine reaction liquid, adjusting a potential of hydrogen (pH) value, adopting an organic solvent to extract, dewatering extraction liquid to form the 2-chlorine-3-aminopyridine hydrochloride with hydrogen chloride, and stirring to crystallize at the appropriate temperature to achieve the 2-chlorine-3-aminopyridine hydrochloride with the purity >= 99%. Impurities of 2,6-dichloro-3-aminopyridine are smaller than or equal to 0.5%. the preparation method is simple in operation, little in equipment investment, low in cost, small in environment pollution and suitable for industrialization production.
Owner:ABA CHEM NANTONG
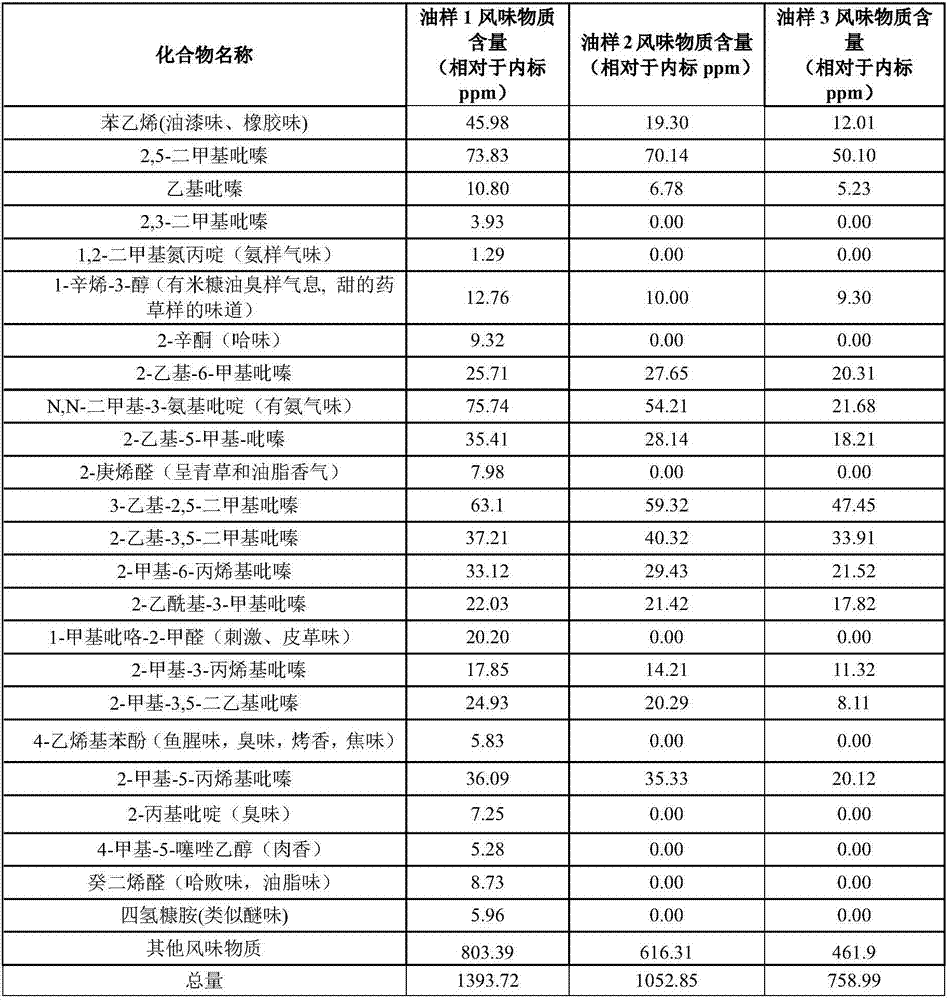
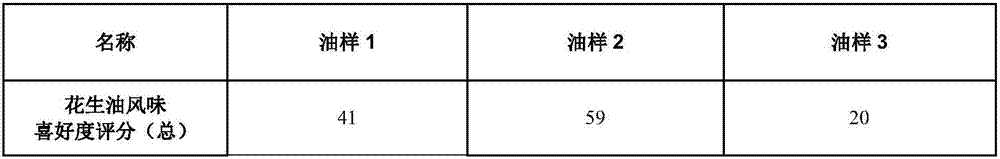
Peanut oil and preparation method thereof
ActiveCN107955703AThe content of bad flavor substances is reducedRaise the ratioFatty-oils/fats refiningFatty-oils/fats productionFlavor3-Aminopyridine
The invention relates to peanut oil with excellent flavor. Based on the total weight of the peanut oil, the content of styrene in the peanut oil is 15-30ppm, and the content of N,N-dimethyl-3-aminopyridine is 40-70ppm. The invention also relates to a preparation method of the peanut oil. The preparation method comprises the steps of adding an adsorbent into peanuts before the peanuts are squeezedfor taking the oil. According to the invention, the peanut oil prepared by the method can improve the ratio of pyrazine flavor substances while reducing bad flavor, and therefore the flavor of the peanut oil is more pure.
Owner:WILMAR SHANGHAI BIOTECH RES & DEV CENT
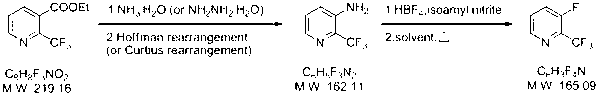
Synthesizing method of 2-trifluoromethyl-3-fluoropyridin
ActiveCN102977009ALow costSimple and fast operationOrganic chemistryTrifluoromethylationChemical industry
The invention relates to the field of medicine and chemical industry. Aims at solving problems of high trifluoromethylation reagent toxicity, or high cost, complicated reaction, and low conversion rate existing in synthesizing reactions of trifluoromethylation similar compounds, the invention provides a synthesizing method of 2-trifluoromethyl-3-fluoropyridin. The method comprises the steps that: (2) with 2-trifluoromethyl ethyl nicotinate as a raw material, 2-trifluoromethyl-3-aminopyridine is produced through a reaction; and (2) the product obtained in the step (1) is subjected to a reaction with fluoboric acid, such that 2-trifluoromethyl-3-fluoropyridin is obtained. According to the invention, the raw materials have low cost, and are easy to obtain. Also, post treatment is simple. The method is suitable for laboratory small-scale preparation, and is suitable for large-scale industrialized production.
Owner:HANGZHOU ALLSINO CHEM
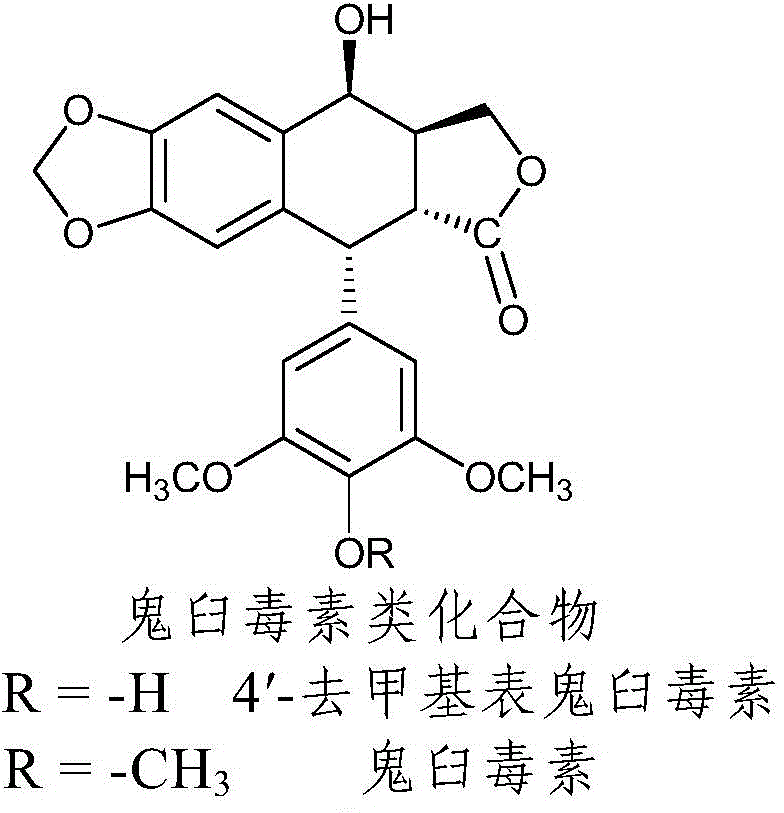
Nitrogen-substituted podophyllotoxin derivative with anti-tumor activity and preparation method and use thereof
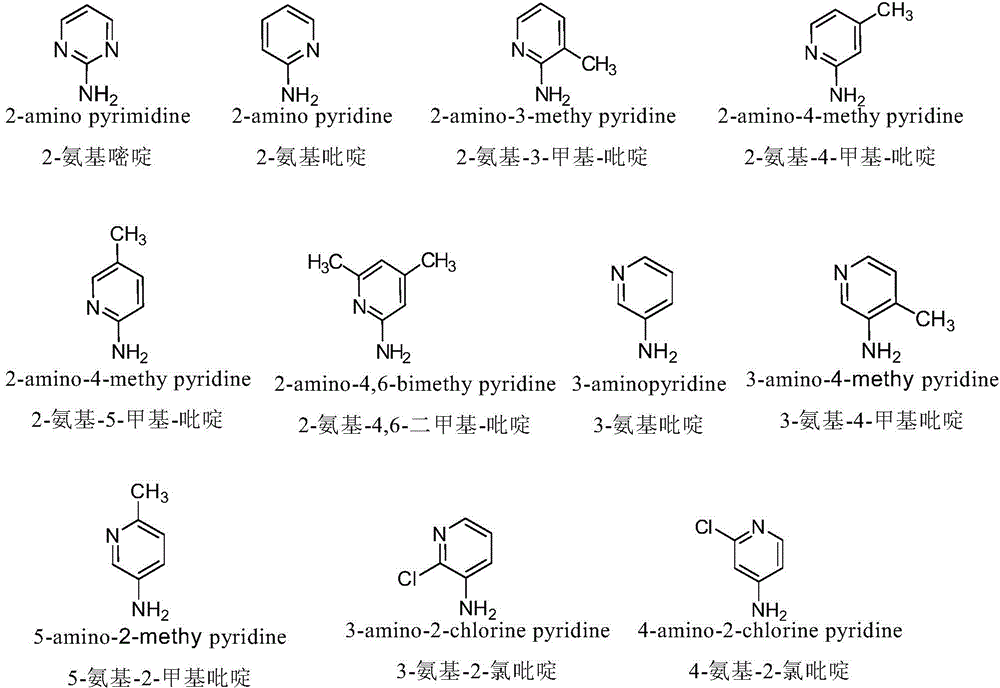
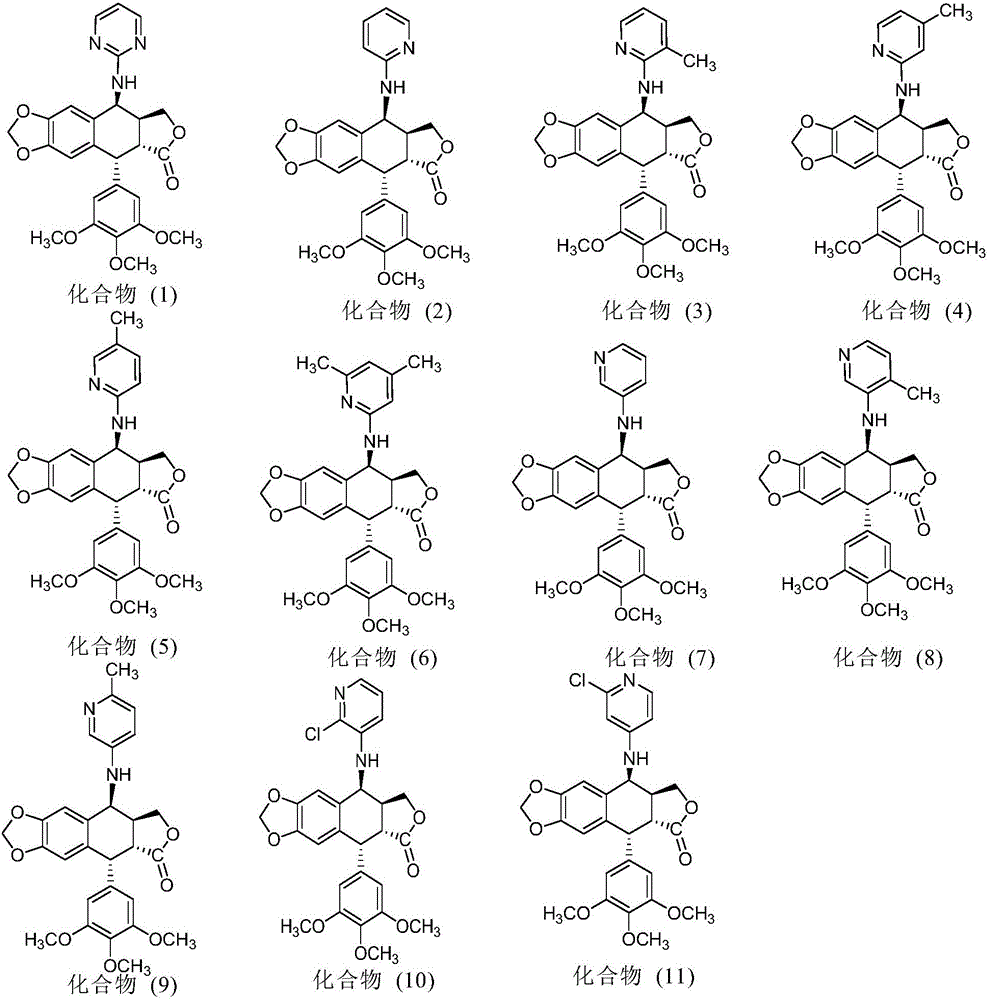
InactiveCN103601732AGood antitumor activityImprove anti-tumor activityOrganic active ingredientsOrganic chemistryTumor cells2-Methylpyridine
The invention discloses a nitrogen-substituted podophyllotoxin derivative with anti-tumor activity and a preparation method and use thereof. According to the method, 2-aminopyrimidine, 2-aminopyridine, 2-amino-3-methylpyridine, 2-amino-4-methylpyridine, 2-amino-5-methylpyridine, 2-amino-4,6-dimethyl pyridine, 3-aminopyridine, 3-amino-4-methylpyridine, 5-amino-2-methylpyridine, 3-amino-2-chloropyridine or 4-amino-2-chloropyridine is respectively introduced to an activated C-ring fourth position of a podophyllotoxin compound through nitrogen substitution reaction, so as to obtain the nitrogen-substituted podophyllotoxin derivative, represented by a formula (V) shown in the specification, with excellent anti-tumor activity. The nitrogen-substituted podophyllotoxin derivative disclosed by the invention acts on tumor cells through multiple ways and multiple target points, and the anti-tumor activity of the nitrogen-substituted podophyllotoxin derivative is remarkably improved compared with that of the podophyllotoxin compound. The compound disclosed by the invention can be used for preparing anti-tumor drugs and is clinically applied to anti-tumor treatment.
Owner:HUBEI UNIV OF TECH
A kind of preparation method of 2,3-dichloropyridine
Owner:NANTONG TENDENCI CHEM
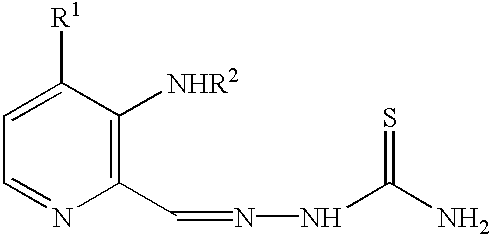
Use of composition containing 3-aminopyridine-2-formaldehyde thiosemicarbazone in treatment of cancers
InactiveCN102210698ANo damageHeavy metal active ingredientsAntineoplastic agents3-AminopyridinePyridine
The invention provides a medicinal composition, which contains an effective dose of 3-aminopyridine-2-formaldehyde thiosemicarbazone for treating cancers and a pharmaceutically acceptable carrier. The invention also provides a composition for oral taking, which contains an effective dose of 3-aminopyridine-2-formaldehyde thiosemicarbazone and the acceptable carrier. The invention also provides the medicinal composition for intravenous administration, which contains 3-aminopyridine-2-formaldehyde thiosemicarbazone in an amount which is enough for supplying about 100 to 200mg / m<2> to an acceptor every day. Finally, the invention provides a container which can prevent 3-aminopyridine-2-formaldehyde thiosemicarbazone from decomposing and being damaged in light and contains at least an effective dose of 3-aminopyridine-2-formaldehyde thiosemicarbazone.
Owner:VION PHARMA INC
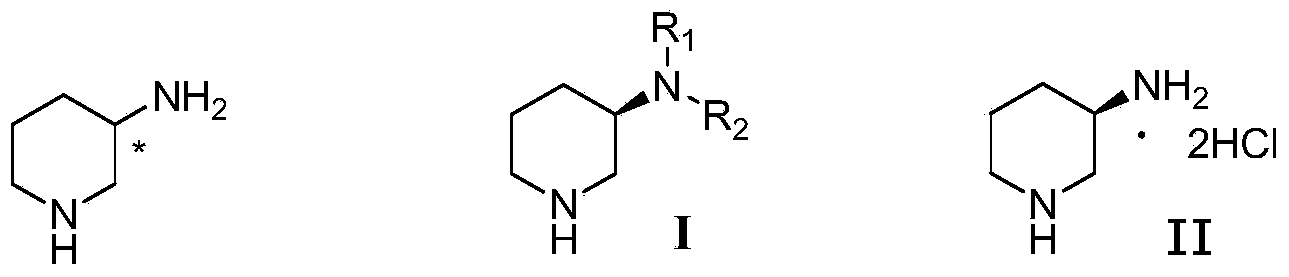
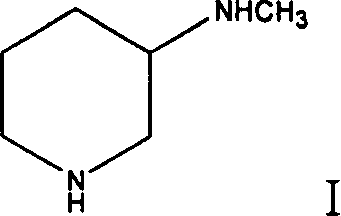
Method for preparing 3-methylamino piperidine and its salt
A process for preparing 3-methylamino piperidine and its salt from 3-aminopyridine includes methylation reaction to obtain 3-methylamino pyridine, reducing itin metallic sodium-alcohol system to obtain 3-methylamino piperdine, and reacting on acid to obtain its salt.
Owner:LIANYUNGANG RUNZHONG PHARMA CO LTD
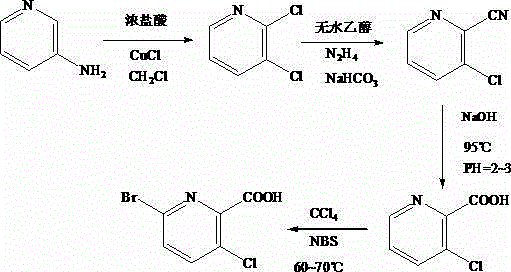
Synthesis method of 3-cloro-5-bromo-2-picolinic acid
The invention discloses a synthesis method of 3-cloro-5-bromo-2-picolinic acid, and belongs to the field of chemosynthesis. According to the method, 3-aminopyridine is used as raw materials; concentrated hydrochloric acid and hydrogen peroxide are added for preparing a diazonium salt solution; CuCl, dichloromethane and concentrated hydrochloric acid are added into a three-mouth flask; the diazonium salt solution is dropwise added into the three-mouth flask; the pH value is regulated; extraction, separation and rotary evaporation are carried out to obtain 2, 3-dichloropyridine; absolute ethyl alcohol is used for dissolution; a hydrazine hydrate solution is dropwise added into the flask; under the nitrogen gas protection, backflow is carried out; 3-cloro-2-cyanopyridine can be obtained; then, through specific conditions, the 3-cloro-2-cyanopyridine is used for producing 3-chloropyridine-2-picolinic acid; then, N-bromo-succinimide is added for performing a bromination reaction to finally obtain the 3-cloro-5-bromo-2-picolinic acid.
Owner:高大元
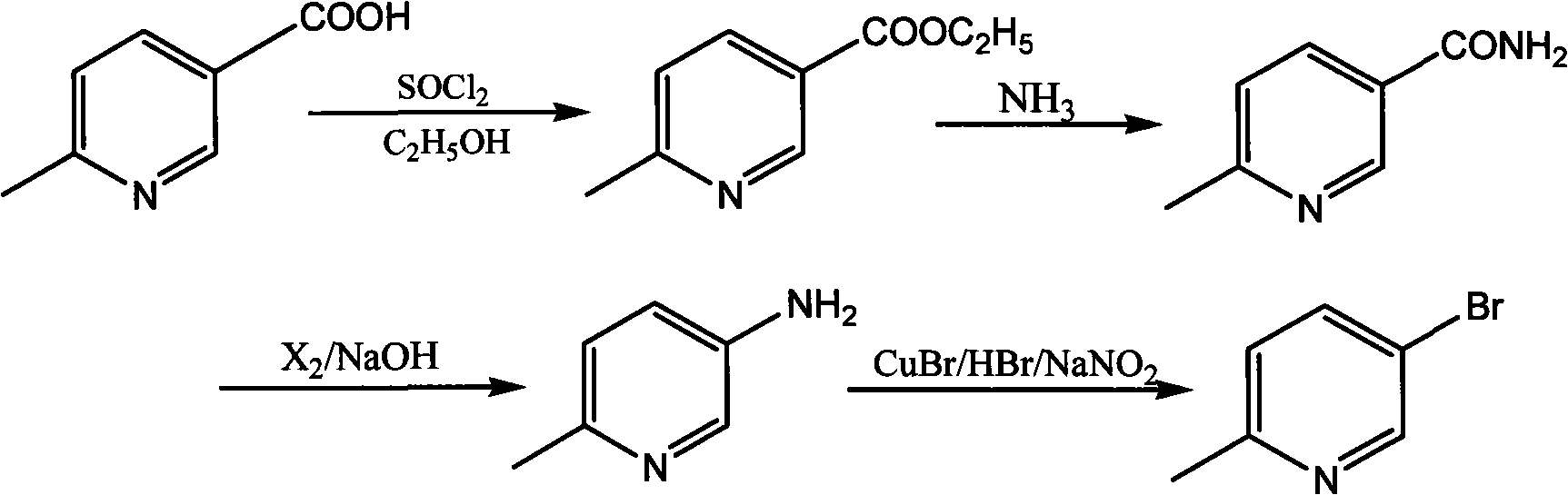
Synthetic method for 5-bromine-2-methylpyridine
InactiveCN101514184AReduce wasteMild reaction conditionsOrganic chemistryPhotochemistryPicolinic acid
The invention discloses a synthetic method for 5-bromine-2-methylpyridine: using 6-methyl-3-picolinic acid as the raw material to react with ethyl alcohol to generate 6-methyl-3-picolinic acid ethyl ester; carrying out ammonolysis reaction by aqueous ammonia on the 6-methyl-3-picolinic acid ethyl ester to generate 6-methyl-3-pyridine carboxamide; carrying out Hofmann degradation reaction to obtain 6-methyl-3-aminopyridine; and finally reacting the 6-methyl-3-aminopyridine with a bromizing reagent to generate 5-bromine-2-methylpyridine. In the method, the process reaction condition is mild, the yield is high, the raw material is available, the cost is lower, and no 3-subsidary products are generated in the whole process, the load of post separation is eliminated and the prospect of industrialization is good.
Owner:NANJING UNIV OF TECH
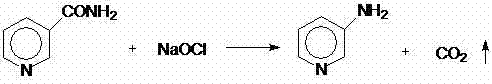
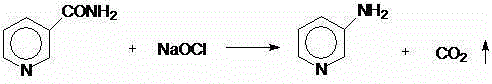
One-step method for preparing 3-aminopyridine
InactiveCN107963990AImprove turnover rateIncrease unit outputOrganic chemistryLiquid temperatureBottle
The invention relates to a one-step method for preparing 3-aminopyridine. Hofmann degradation is directly performed when 3-cyanopyridine is not hydrolyzed into nicotinamide, the reaction time of a reaction device is shortened, reaction steps are decreased, application of energy is decreased, and energy is saved. The method includes the steps: 1) preparing raw materials such as sodium hypochloritesolution with available chlorine of 10%, water, sodium hydroxide solution and 3-cyanopyridine; 2) sequentially placing the sodium hypochlorite solution, the water and the sodium hydroxide solution into a four-port glass reaction bottle, stirring mixture under ice-salt bath, and cooling the mixture to reach the temperature of 0 DEG C; 3) taking 3-cyanopyridine, placing the taken 3-cyanopyridine into a mortar, grinding the 3-cyanopyridine, adding the grinded 3-cyanopyridine into the stirring four-port reaction bottle by a paper adding cylinder, flushing the mortar by the aid of a washing bottleand the 3-cyanopyridine adhered on the glass ports, adding residues into the four-port reaction bottle, heating mixture in the adding process, keeping the ice-salt bath, controlling the adding speed of the 3-cyanopyridine, and enabling the temperature of liquid in the reaction bottle not to exceed 5 DEG C.
Owner:CANGZHOU LINGANG YANUO CHEM CO LTD
Process study for synthesizing 2,3,6-trichloropyridine from nicotinamide
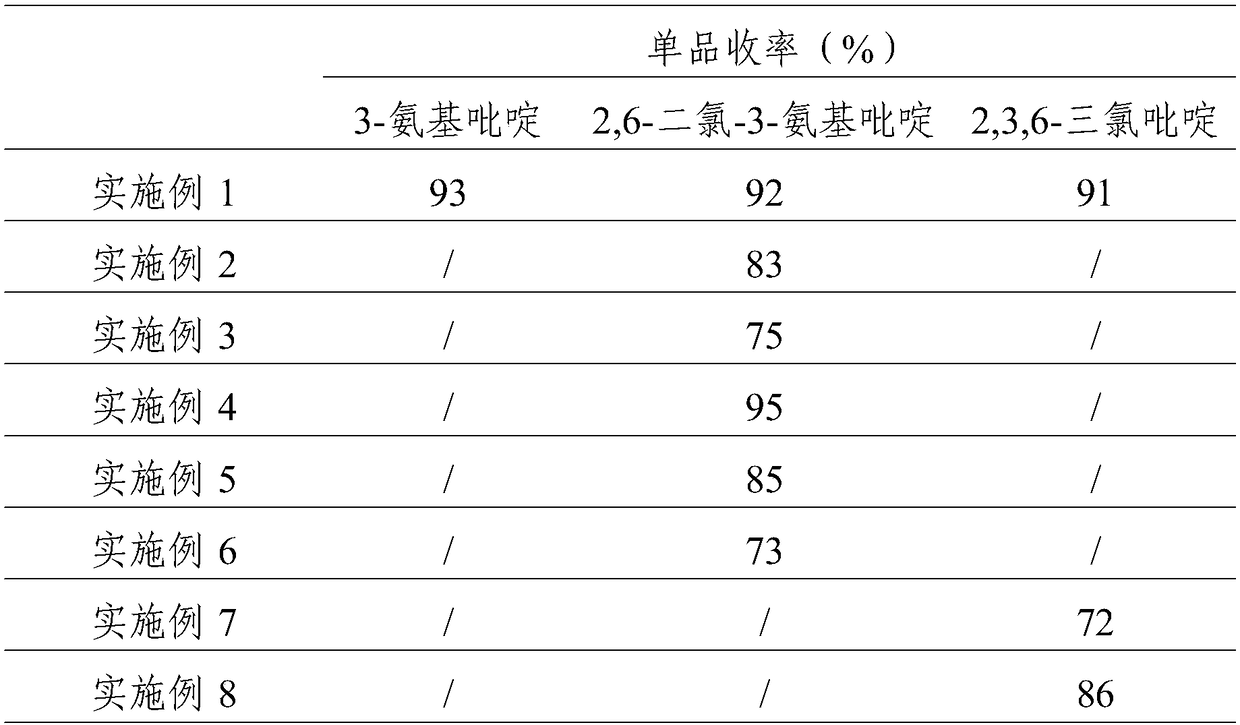
The invention provides a process study for synthesizing 2,3,6-trichloropyridine from nicotinamide. The process study comprises the following steps: with nicotinamide as a raw material, adding a sodiumhypochlorite solution in an alkaline environment to carry out a Hofmann downgrading reaction to obtain 3-aminopyridine; under catalysis of a Lewis acid catalyst, performing chlorination reaction under a concentrated hydrochloric acid / hydrogen peroxide condition to obtain 2,6-dichloro-3-aminopyridine; and reacting in the presence of sodium nitrite under low temperature and strong acid conditions to form a diazonium salt solution; and finally, performing a Sandmeyer reaction to obtain the target product, 2,3,6-trichloropyridine. The process material provided by the invention is simple, easily available and cheap, the reaction condition is simple and easy to operate, the post-treatment is simple, the yield is high, and thus the process has good industrial development prospects.
Owner:ANHUI COSTAR BIOCHEM CO LTD
Method for preparing 2,3-dichloropyridine
The invention discloses a method for preparing 2,3-dichloropyridine with a simple process and a high yield rate. The method is as follows: in a concentrated hydrochloric acid, 3-aminopyridine is used as a starting material, Fe<2+> or Fe <3+> is used as a chlorination catalyst, and a mixture of hydrogen peroxide and hydrochloric acid or chlorine is used as a chlorating agent to conduct a chlorination reaction to chloridize the 3-aminopyridine. The reaction mixtures without being separated are added with Cu<+> or Cu<2+> which serves as a catalyst for diazotization / chlorination reaction and with an aqueous solution sodium nitrite to conduct diazotization / chlorination reaction to prepare 2,3-dichloropyridine by a one-pot process. The 2,3-dichloropyridine is isolated and purified by a normal method with a purity more than 99.2 percent. According to a 3-aminopyridine based calculation, the molar yield rate of the 2,3-dichloropyridine is more than 74.1 percent.
Owner:CANGZHOU LINGANG YANUO CHEM CO LTD
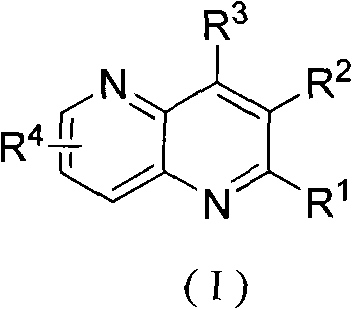
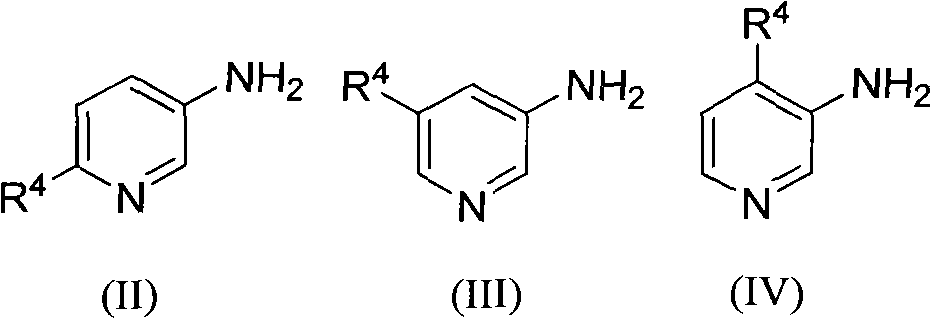
Method for preparing poly-substituted 1, 5-naphthyridine compound
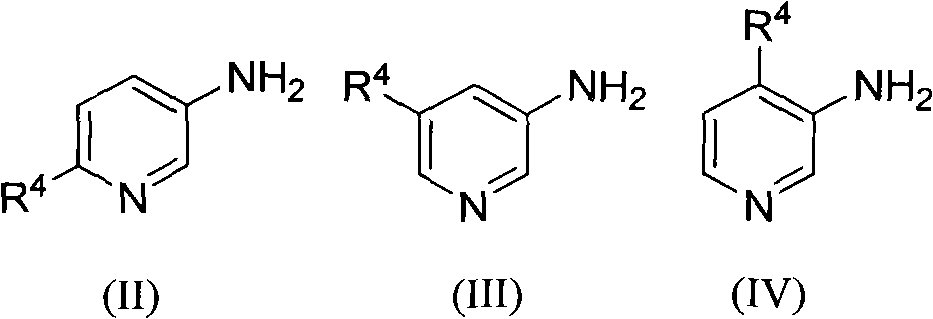
The invention relates to a method for preparing a poly-substituted 1, 5-naphthyridine compound. The method comprises the steps of: mixing 3-aminopyridines compound shown in constitutional formula (II), (III) or (IV) with 2-alkenyl aldehyde or 2-alkenyl ketone; adding green vitriol as a catalyst; carrying out heating reflux reaction in sulphuric acid for 2 to 20 hours; cooling a reaction system till the temperature of the reaction system reaches room temperature and then regulating pH value to be neutral; filtering the reactants and extracting, concentrating, separating and refining the filtrate to obtain the poly-substituted 1, 5-naphthyridine compound shown in constitutional formula (I). The method can be applied to various substrates and materials are highly available; poly-substituted 1, 5-naphthyridine compound banks with diverse structures can be synthesized by optimizing and regulating substrates involved in the reaction, and the compounds can be widely applied to the fields such as pharmacochemistry, biomedicine, materials science and the like.
Owner:合肥华纳生物医药科技有限公司
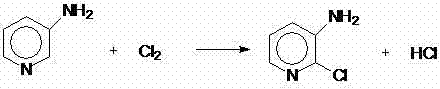
Preparation method of 2-chloro-3-aminopyridine
ActiveCN102532010AGuaranteed to getReduce manufacturing costOrganic chemistryAlkyl transfer3-Aminopyridine
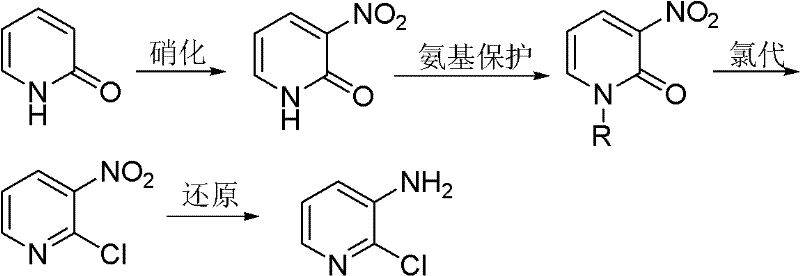
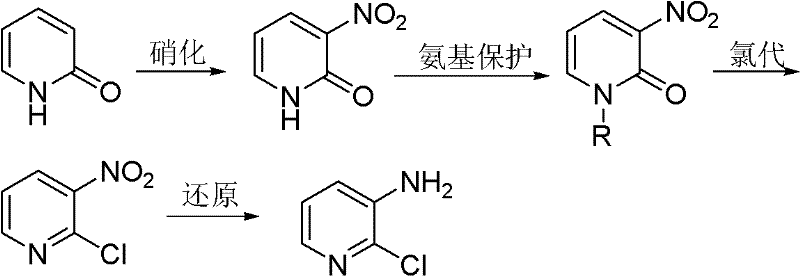
The invention discloses a new preparation method of 2-chloro-3-aminopyridine. The method comprises the following steps of: using 2-pyridone as the raw material to perform a nitration reaction and an N-alkylation reaction for amino protection to obtain N-alkyl-3-nitro-2-pyridone, then adding a proper chlorinating agent to perform a directional chlorination reaction and a dealkylation protection reaction to obtain 2-chloro-3-nitropyridine, and finally reducing to obtain 2-chloro-3-aminopyridine. The method has cheap and available raw materials, high selectivity, fewer by-products and high yield.
Owner:SINO HIGH CHINA
Preparation method of 2-chloro-3-aminopyridine
InactiveCN102153509AMild responseSimple and fast operationOrganic chemistryOrganic solvent3-Aminopyridine
The invention belongs to the field of fine chemical industry, and particularly relates to a preparation method of 2-chloro-3-aminopyridine. The method comprises the following steps: adding 2-chloro-3-nitropyridine into water, and dropwisely adding a sodium sulfide water solution to carry out reaction; after the reaction finishes, cooling to precipitate a solid, adding dilute hydrochloric acid to dissolve the solid, filtering, regulating the pH value of the filtrate to 9-10 with an NaOH water solution, and precipitating crystals, thereby obtaining the 2-chloro-3-aminopyridine. The preparation method of 2-chloro-3-aminopyridine has the advantages of mild reaction, high product yield and the like, is simple to operate, and is suitable for industrial production; no organic solvent is used in the reaction process, thereby being beneficial to clean production; and the raw materials are cheap and accessible, thereby lowering the production cost.
Owner:UNIV OF JINAN
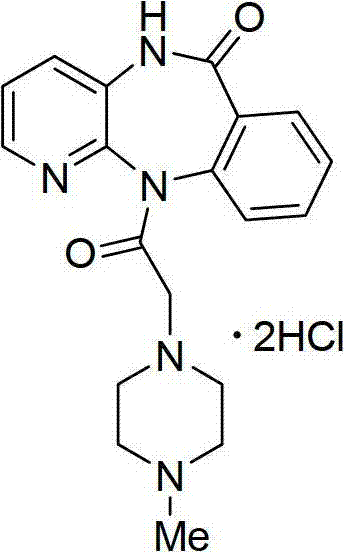
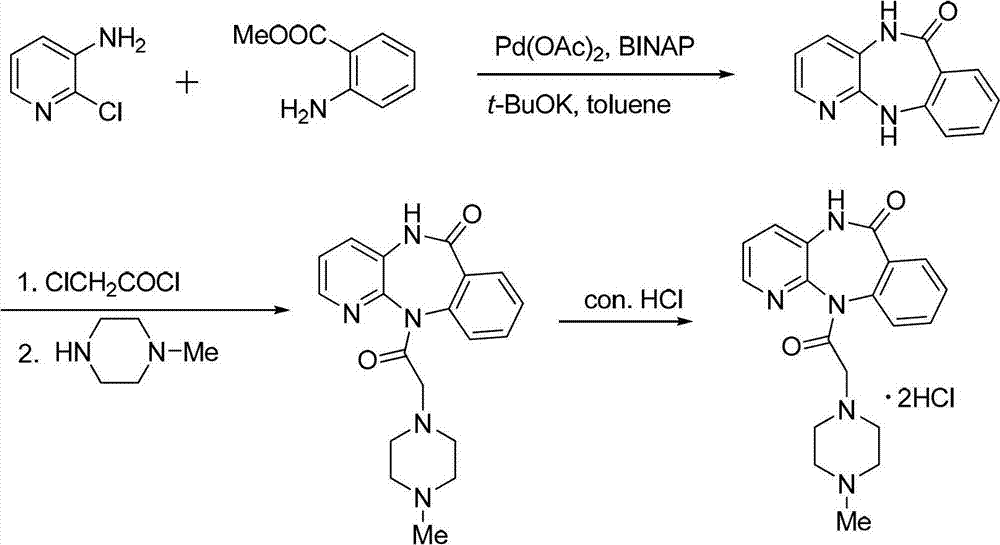
Method for synthesizing pirenzepine hydrochloride
InactiveCN103044419ASimple ingredientsMild reaction conditionsOrganic chemistrySolvent3-Aminopyridine
The invention discloses a method for synthesizing pirenzepine hydrochloride, comprising the following steps of: (1) by taking 2-chloro-3-aminopyridine and methyl anthranilate as raw materials, a palladium compound as a catalyst, a phosphorous compound as a ligand and a benzene solvent as a solvent, reacting all the materials under the action of alkali to obtain a cyclization midbody benzodiazepinone; (2) carrying out acylation reaction on the cyclization midbody benzodiazepinone and chloroacetyl chloride in the solvent under the action of alkali to obtain N-chloracetyl-benzodiazepinone, and reacting the N-chloracetyl-benzodiazepinone with N-methyl piperazine to obtain pirenzepine; and (3) heating the pirenzepine in the presence of concentrated hydrochloric acid to obtain pirenzepine hydrochloride. The method provided by the invention is characterized in that palladium is employed as the catalyst, the reaction raw materials are simple, the reaction conditions are mild, and the reaction yield is high, which can be 95 or higher. The method is low in associated reaction corrosion, low in three-waste treatment load, simple in process, low in cost and capable of meeting the industrial production requirements.
Owner:SUZHOU HOMESUN PHARMA
Novel method for synthesizing 2,5-dichloro-3-fluoropyridine
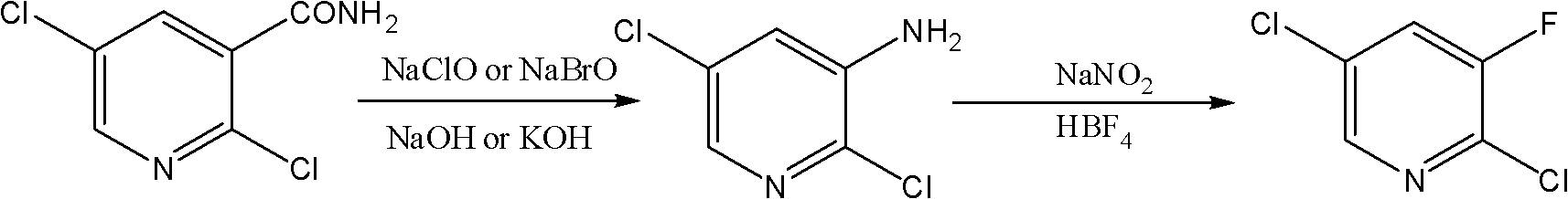
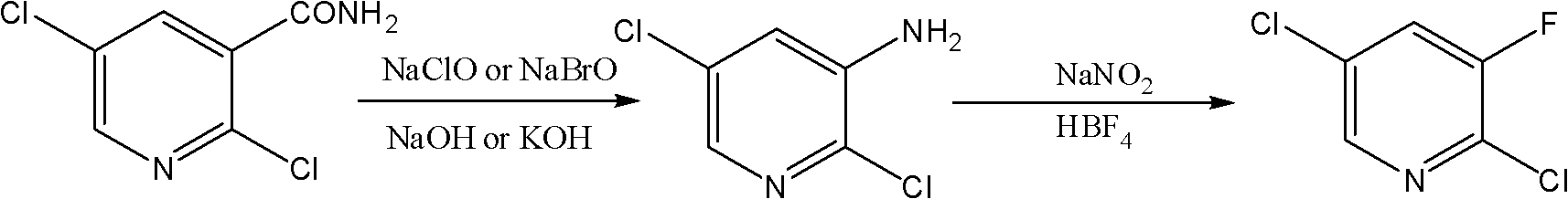
The invention belongs to the field of chemical industry and discloses a novel method for synthesizing 2,5-dichloro-3-fluoropyridine. The method comprises the following steps of: performing Hofmann degradation on 2,5-dichloro-nicotinamide under the action of sodium hypobromite or sodium hypochlorite to obtain 2,5-dichloro-3-aminopyridine, and performing diazotization reaction on the 2,5-dichloro-3-aminopyridine, fluoboric acid and sodium nitrite to obtain the 2,5-dichloro-3-fluoropyridine. By the method, the yield of the 2,5-dichloro-3-fluoropyridine is greatly improved, and the total yield of the 2,5-dichloro-3-fluoropyridine can reach over 67 percent at most; and expensive 2,3,5-trichloropyridine is not used, and an expensive fluridizer is not used, so that the synthetic cost of the 2,5-dichloro-3-fluoropyridine is greatly reduced. Moreover, reaction steps are simple, the reaction process is mild, and the method is easy to control.
Owner:NANJING REDSUN BIOCHEM CO LTD
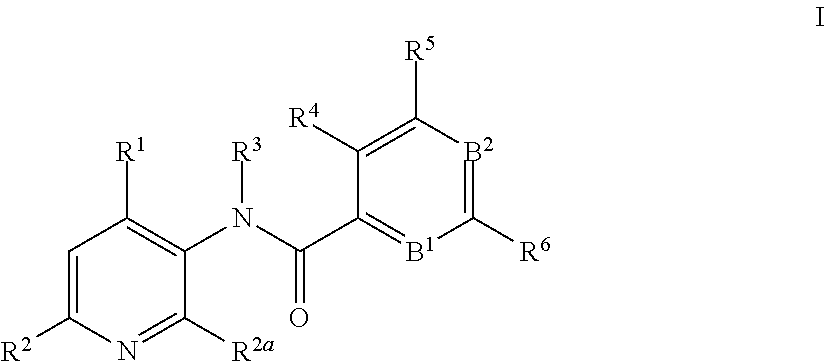
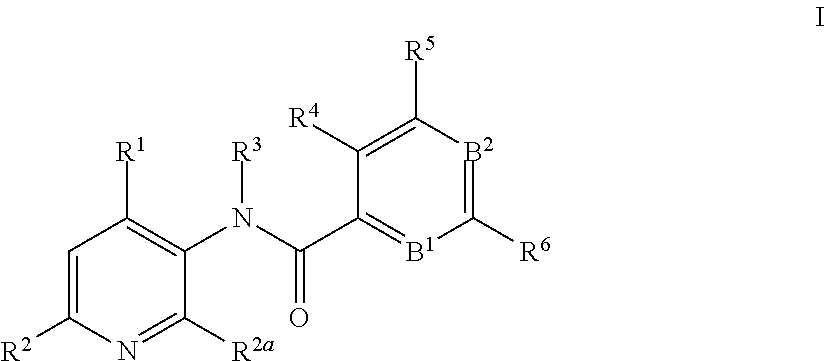
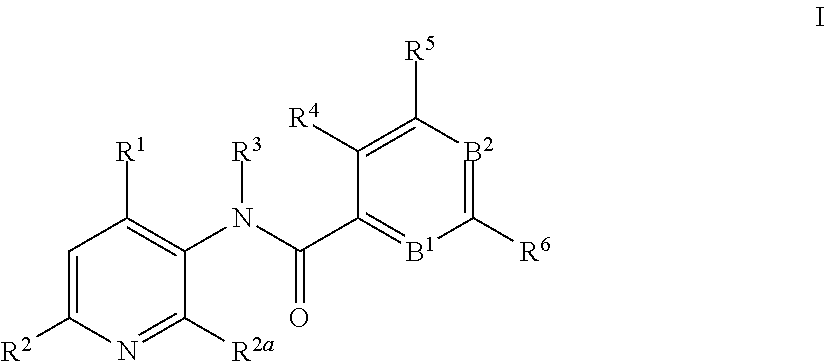
3-amino-pyridines as GPBAR1 agonists
This invention relates to novel 3-aminopyridines of the formulawherein B1, B2 and R1 to R6 are as defined in the description and in the claims, as well as pharmaceutically acceptable salts thereof. These compounds are GPBAR1 agonists and can be used as medicaments for the treatment of diseases such as type II diabetes.
Owner:F HOFFMANN LA ROCHE INC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com