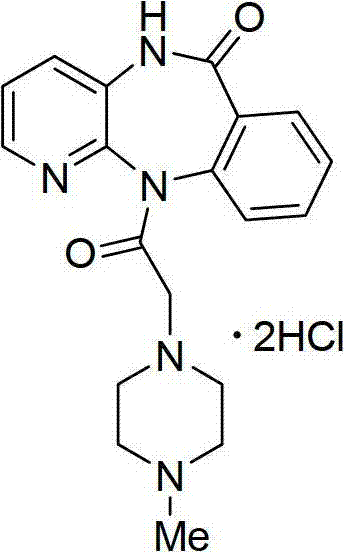
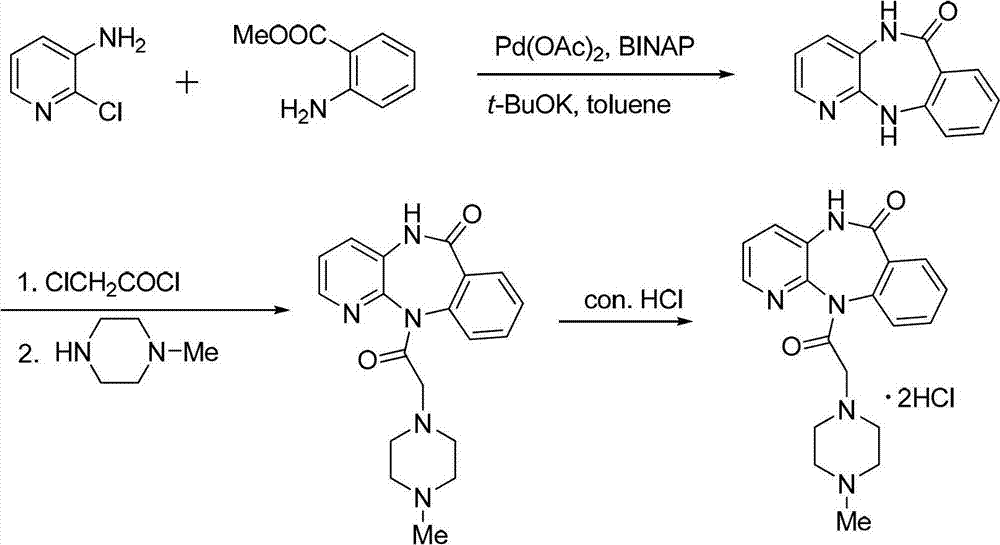
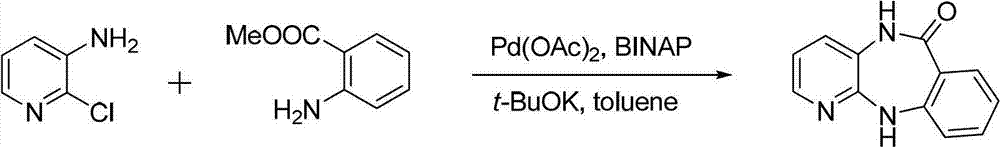Method for synthesizing pirenzepine hydrochloride
A technology for pirenzepine hydrochloride and a mixture, which is applied in the field of chemical synthesis, can solve the problems of unfavorable industrialized production and high reaction temperature, and achieves the effects of being beneficial to large-scale production, high yield, and low burden on three wastes treatment
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] (1)
[0037]
[0038]Add 300 ml of toluene into the reaction vessel, dissolve 257 g of 2-chloro-3-aminopyridine in the toluene, and stir evenly with a mechanical stirrer. Slowly add 292 grams of potassium tert-butoxide, the addition speed is 5% dosage / minute, and stir evenly. Then the methyl anthranilate of 393 grams is added in the reaction solvent with the mode of dripping, and the rate of addition is 3% consumption / minute. . React at 50°C for 1 hour. After the reaction, add 400ml of toluene, 4.49g of palladium acetate and 12.5g of binaphthyl diphenylphosphine, raise the reaction temperature to 110°C for 24 hours. After the reaction was finished, the solvent was evaporated under reduced pressure, and the white solid was obtained by suction filtration, and then recrystallized with 600 ml of a mixed solvent of 50% acetone and water, filtered by suction, washed with petroleum ether, and dried to obtain the cyclized intermediate benzodiazepine 401 grams of Zadrowne...
Embodiment 2
[0044] Compared with Example 1, the only difference is that in this example, the catalyst in step (1) is palladium acetate, and the amount of catalyst used is 3% of 2-chloro-3-aminopyridine in terms of moles; the ligand is triphenyl Phosphine, the amount of ligand is 2 times that of the palladium catalyst in terms of moles; the alkali is potassium carbonate, and its molar amount is 2 times that of 2-chloro-3-aminopyridine, and the rate of addition is 5% consumption / minute; Methyl aminobenzoate is added dropwise in the reaction solvent, and the drop rate is 4% consumption / minute. The solvent is toluene; the reaction temperature is 110°C, and the reaction time is 24h.
[0045] In step 1, the product yield is 71%, and the purity is 99%. The NMR data of product are with embodiment 1.
[0046] In the step (2), the solvent is dioxane; the base is potassium carbonate, and the molar amount of the base is twice that of the cyclization intermediate benzodiazepine.
[0047] In step 2,...
Embodiment 3
[0051] Compared with Example 1, the only difference is that in this example, the catalyst in step (1) is palladium trifluoroacetate, and the amount of catalyst used is 1% of 2-chloro-3-aminopyridine in terms of moles; the ligand is three Cyclohexylphosphine, tri-p-tolylphosphine, the amount of ligand is 1.5 times that of the palladium catalyst in terms of moles; the alkali is an equal mixture of sodium carbonate and sodium hydroxide, and the molar amount of the alkali is 2-chloro-3- 1 time of aminopyridine; the dropping speed is 6% dosage / minute; methyl anthranilate is added to the reaction solvent in a dropwise manner, and the dropping speed is 2% dosage / minute. The solvent is o-xylene; the reaction temperature is 90°C, and the reaction time is 36h.
[0052] The product yield of step 1 is 63%, and the purity is 99%. The NMR data of product are with embodiment 1.
[0053] In the step (2), the solvent is dichloromethane; the base is sodium carbonate, and the molar amount of t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com