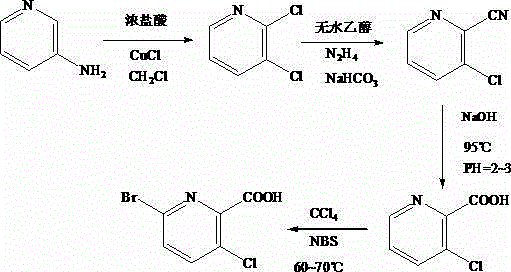
Synthesis method of 3-cloro-5-bromo-2-picolinic acid
A technology of picolinic acid and synthesis method, which is applied in the direction of organic chemistry and can solve problems such as not being able to meet market development requirements
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0019] First, add 35g of 3-aminopyridine to a 1L three-necked flask, and add 300mL of hydrochloric acid with a mass concentration of 60% into the flask, and stir it with a machine to completely dissolve it. Add 100mL of hydrogen peroxide with a mass fraction of 10%, and keep stirring at this temperature for 2h. After stirring, lower the temperature to -5°C, and then add 100g of NaNO 2 , and continue to stir for 25min to obtain a diazonium salt solution; take another 1L three-necked flask, add 20gCuCl, 250mL dichloromethane and 5mL concentrated hydrochloric acid with a mass fraction of 70% to it, and then dissolve the above-mentioned solution under stirring Slowly add the diazonium salt solution into the flask, and control the temperature at 15°C. After the addition is completed, keep stirring at this temperature for 20min, then raise the temperature to 30°C, and continue the reaction for 1h. After the reaction is completed, cool to room temperature and use NaOH solution Adjust...
example 2
[0021] First, add 40g of 3-aminopyridine to a 1L three-necked flask, and add 350mL of hydrochloric acid with a mass concentration of 65% into the flask, stir it mechanically to dissolve it completely, then place it in a water bath, control the temperature at 6°C, and pour it into the flask. Add 105mL of hydrogen peroxide with a mass fraction of 11%, and keep stirring at this temperature for 2.5h. After stirring, lower the temperature to -5°C, and then add 105g of NaNO 2, and continue to stir for 28min to obtain a diazonium salt solution; take another 1L three-neck flask, add 21gCuCl, 275mL dichloromethane and 6mL concentrated hydrochloric acid with a mass fraction of 70% to it, and then dissolve the above-mentioned solution under stirring The obtained diazonium salt solution was slowly added to the flask, and the temperature was controlled at 17°C. After the addition was completed, keep stirring at this temperature for 30 minutes, then raise the temperature to 35°C, and continu...
example 3
[0023] First, add 45g of 3-aminopyridine to a 1L three-necked flask, and add 400mL of hydrochloric acid with a mass concentration of 70% to the flask, stir it mechanically to dissolve it completely, then place it in a water bath, control the temperature at 7°C, and pour it into the flask. Add 110mL of hydrogen peroxide with a mass fraction of 12%, and keep stirring at this temperature for 3h. After stirring, lower the temperature to -5°C, and then add 110g of NaNO 2 , and continue to stir for 30min to obtain a diazonium salt solution; take another 1L three-neck flask, add 22gCuCl, 300mL dichloromethane and 7mL concentrated hydrochloric acid with a mass fraction of 70% to it, and then dissolve the above-mentioned solution under stirring. Slowly add the obtained diazonium salt solution into the flask, and control the temperature at 20°C. After the addition is completed, keep stirring at this temperature for 20-40min, then raise the temperature to 40°C, and continue the reaction f...
PUM
| Property | Measurement | Unit |
|---|---|---|
| quality score | aaaaa | aaaaa |
| quality score | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com