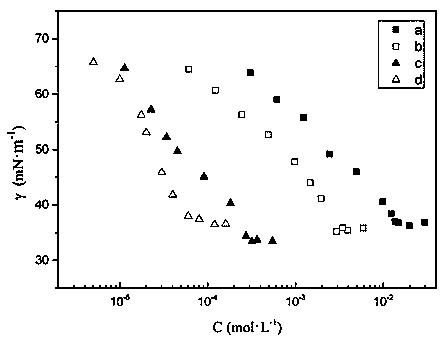
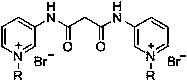
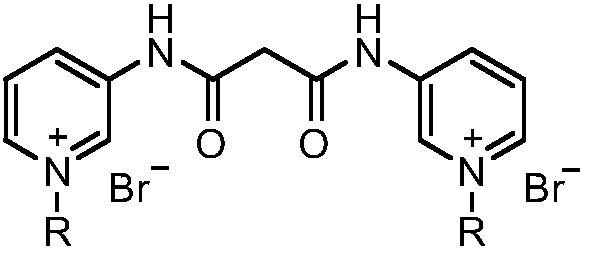
Pyridine bis-quaternary ammonium salt surfactant and preparation method and application thereof
A surfactant and biquaternary ammonium salt technology, applied in the directions of botanical equipment and methods, applications, chemical instruments and methods, can solve the problems of small scale, complex synthesis process, etc., and achieve simple preparation, easy availability of raw materials, Excellent antibacterial effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] Embodiment 1: N bromide, the preparation of N'-bis (1-n-octyl-3-pyridyl) malonamide diquaternary ammonium salt (surfactant a)
[0024]
[0025] (1) Preparation of N, N'-bis(3-pyridyl)malonamide: in a reaction flask, add 3-aminopyridine (7.05g, 75mmol), diethyl malonate (4.00g, 25mmol) and 10mL N, N-dimethylformamide, install a water separator, start stirring, and heat the reaction. During the reaction process, the low-boiling ethanol generated by the reaction was separated with a water separator, and finally the reaction temperature was reached to 140°C for 7 hours, cooled, filtered, washed with ethyl acetate, and dried to obtain 4.16g N, N'-bis( 3-pyridyl)malonamide, yield 65%, melting point: 215-218°C. 1 H NMR (600MHz, DMSO-d 6 ,ppm)δ:10.43(s,2H),8.75(d,J=2.3Hz,2H),8.29(m,2H),8.06(m,2H),7.37(m,2H),3.56(s,2H ).
[0026] (2) In the reaction flask, add N,N'-bis(3-pyridyl)malonamide (2.05g, 8mmol) and n-octane bromide (15.45g, 80mmol), heat to 110°C for 7 hours , ...
Embodiment 2
[0027] Embodiment 2: N bromide, the preparation of N'-two (1-n-decyl-3-pyridyl) malonamide diquaternary ammonium salt (surfactant b)
[0028]
[0029] (1) Preparation of N, N'-bis(3-pyridyl) malonamide: in a reaction flask, add 3-aminopyridine (9.41g, 100mmol), diethyl malonate (4.00g, 25mmol) and 10mL N, N-dimethylformamide, install a water separator, start stirring, and heat the reaction. During the reaction process, the ethanol with a low boiling point generated by the reaction was separated with a water separator, and finally the reaction temperature reached 150°C for 6 hours, cooled, filtered, washed with ethyl acetate, and dried to obtain 4.29g N, N'-bis(3 -pyridyl)malonamide, yield 67%, melting point: 215-218°C. 1 H NMR (600MHz, DMSO-d 6 ,ppm)δ:10.43(s,2H),8.75(d,J=2.3Hz,2H),8.29(m,2H),8.06(m,2H),7.37(m,2H),3.56(s,2H ).
[0030] (2) In the reaction flask, add N,N'-bis(3-pyridyl)malonamide (2.05g, 8mmol) and brominated n-decane (17.69g, 80mmol), heat to 120°C for ...
Embodiment 3
[0031] Embodiment 3: N bromide, the preparation of N'-bis (1-n-dodecyl-3-pyridyl) malonamide diquaternary ammonium salt (surfactant c)
[0032]
[0033] (1) Preparation of N, N'-bis(3-pyridyl) malonamide: in a reaction flask, add 3-aminopyridine (9.41g, 100mmol), diethyl malonate (4.00g, 25mmol) and 11.25mLN, N-dimethylformamide, install a water trap, start stirring, and heat to react. During the reaction process, the low-boiling ethanol generated by the reaction was separated with a water separator, and finally the reaction temperature was reached at 140°C for 7 hours, cooled, filtered, washed with ethyl acetate, and dried to obtain 4.06g N, N'-bis( 3-pyridyl)malonamide, yield 63.5%, melting point: 215-218°C. 1 H NMR (600MHz, DMSO-d 6 ,ppm)δ:10.43(s,2H),8.75(d,J=2.3Hz,2H),8.29(m,2H),8.06(m,2H),7.37(m,2H),3.56(s,2H ).
[0034](2) In the reaction flask, add N,N'-bis(3-pyridyl)malonamide (2.05g, 8mmol) and n-dodecane bromide (15.95g, 64mmol), and heat to 120°C to react 6 ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com