Preparation method for 2,3-dichloropyridine
A technology of dichloropyridine and aminopyridine, which is applied in the field of preparation of 2,3-dichloropyridine, can solve the problems of complex reaction principle, many side reactions, and difficult yield, so as to shorten the preparation process and reduce waste liquid The effect of producing and reducing production costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
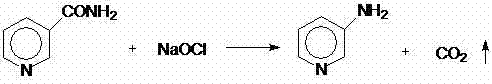
[0037] Preparation of 3-aminopyridine: Add 150 ml of water into a 1000 ml four-necked round bottom flask equipped with a stirring and thermometer, add 36.6 g (0.3 mol) of nicotinamide under stirring, and cool down to 0-5°C after completely dissolving , and then 272 g (0.33 mol) of 9.05% sodium hypochlorite solution was added dropwise in the range of 0-5°C during 30-60 minutes, and stirred for 30 minutes after the dropwise addition was completed. At the same temperature, 48 grams (0.6 mol) of 50% liquid caustic soda was added dropwise, and the reaction was stirred for 30 minutes.
[0038] In another 1000 ml four-necked round bottom flask equipped with stirring, thermometer and condenser tube, add 240 ml of water, heat up to 90-100°C, and add dropwise at 0 during this temperature range and 30-45 minutes The above reaction solution stored at -5°C was continued for 2 hours after the dropwise addition. After the reaction, the weight of the reaction solution was 747 grams. The conte...
Embodiment 2
[0045] Embodiment 2: the preparation of 3-aminopyridine
[0046] Method 1: Add 300 ml of water into a 1000 ml four-necked round bottom flask equipped with stirring, thermometer and condenser tube, add 73.3 g (0.6 mol) of accurately measured nicotinamide under stirring, and cool down to 0- At 5°C, 26.7 g (0.2 mol) of 30% liquid caustic soda was added dropwise. Then control the temperature of the material within the range of 0-5°C, add dropwise 545 g (0.66 mol) of 9.05% sodium hypochlorite solution, and stir for 30 minutes after the dropwise addition. Within the same temperature index, the dropwise concentration is 213 grams (1.6mol) of 30% liquid caustic soda, and the material is controlled within 0-8°C after the dropwise addition is completed. Stir for 30 minutes to raise the temperature, raise the temperature to 70-80°C, and keep it warm for 2 hours. After the reaction, weigh the weight of the reaction solution (1184 grams), analyze the content of 3-aminopyridine with liquid...
Embodiment 3
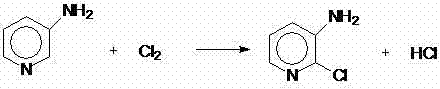
[0050] Preparation of 2-chloro-3-aminopyridine: Add 360 grams of industrial hydrochloric acid (content: 30.5%, 3mol), 0.3mol (28.2 grams) of 3-aminopyridine into a 1000ml four-neck round-bottomed flask equipped with stirring and a thermometer. Pyridine, stir to dissolve, add 27% hydrogen peroxide 45.4g (0.36mol) dropwise at 10-15°C, dropwise for 1.5-2 hours, keep warm at 10-15°C for reaction, after 3.5 hours of reaction, the reaction is over, add 10g of sub Sodium bisulfate until the starch potassium iodide test paper does not show blue, using liquid chromatography, external standard method analysis, the molar yield of 2-chloro-3-aminopyridine is 86.5% (based on 3-aminopyridine).
[0051] The following preparation method is the same as in Example 1. In this example, the content of 2,3-dichloropyridine: 98.8%, and the molar yield: 69.5%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com