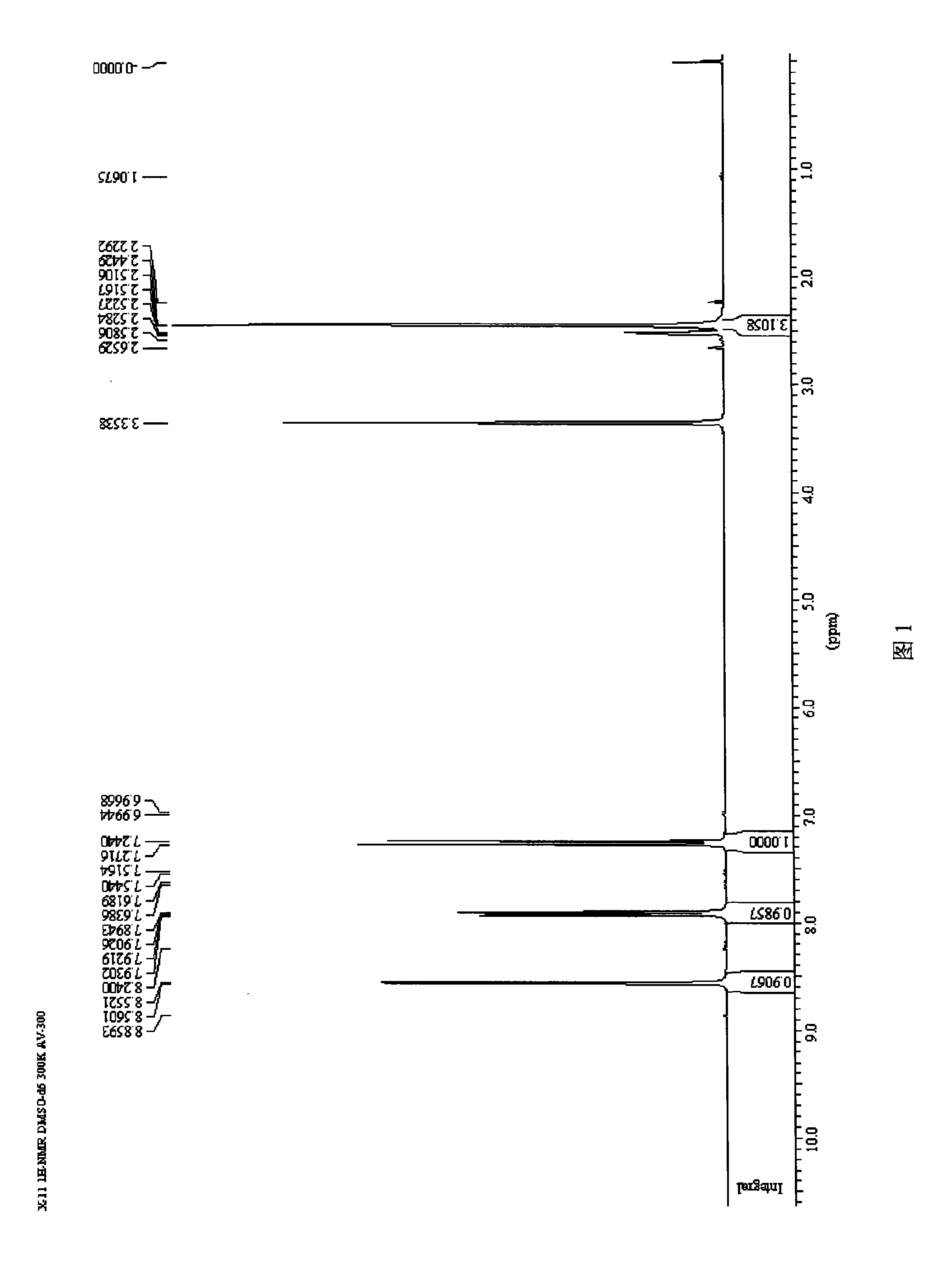
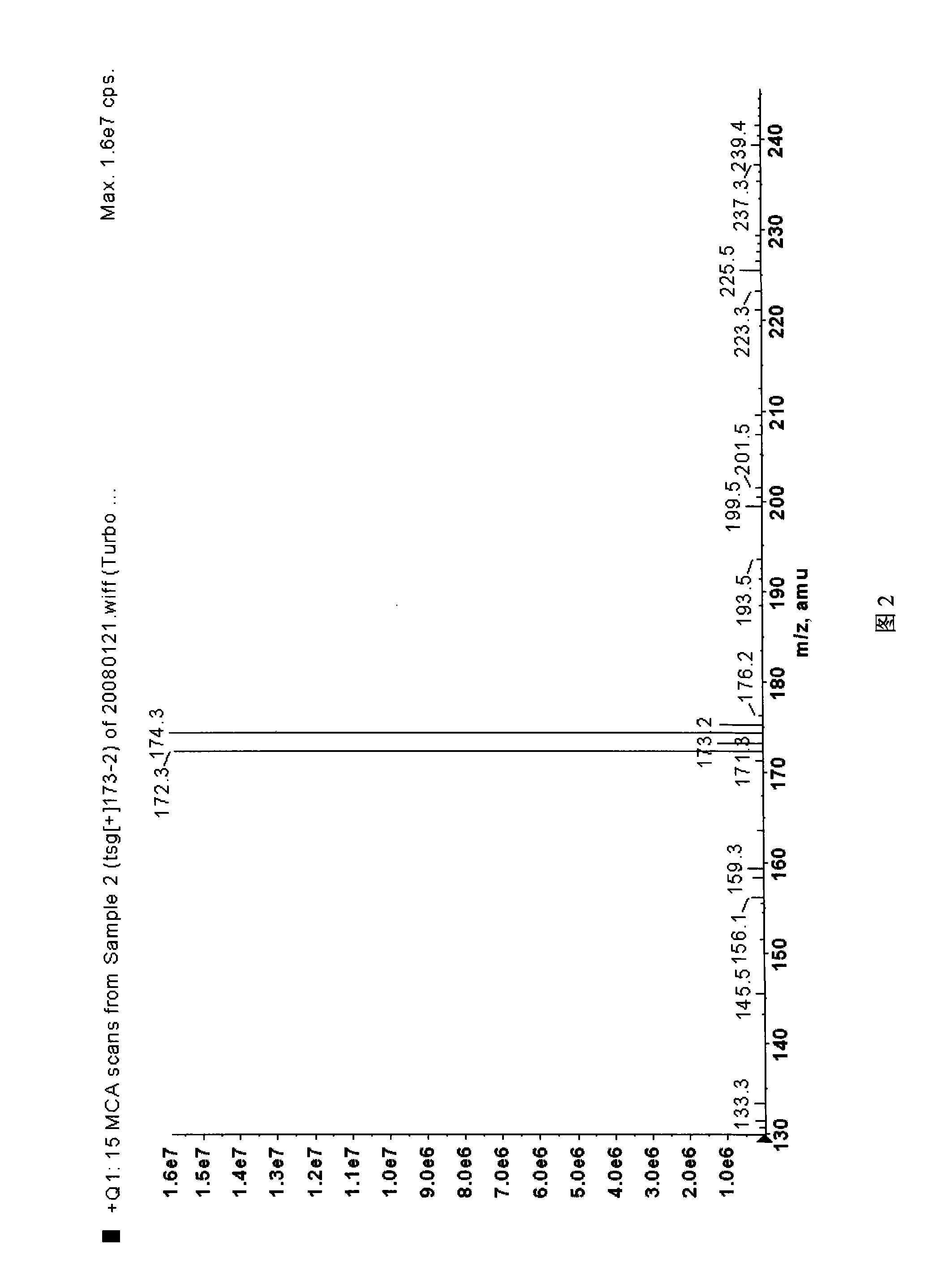
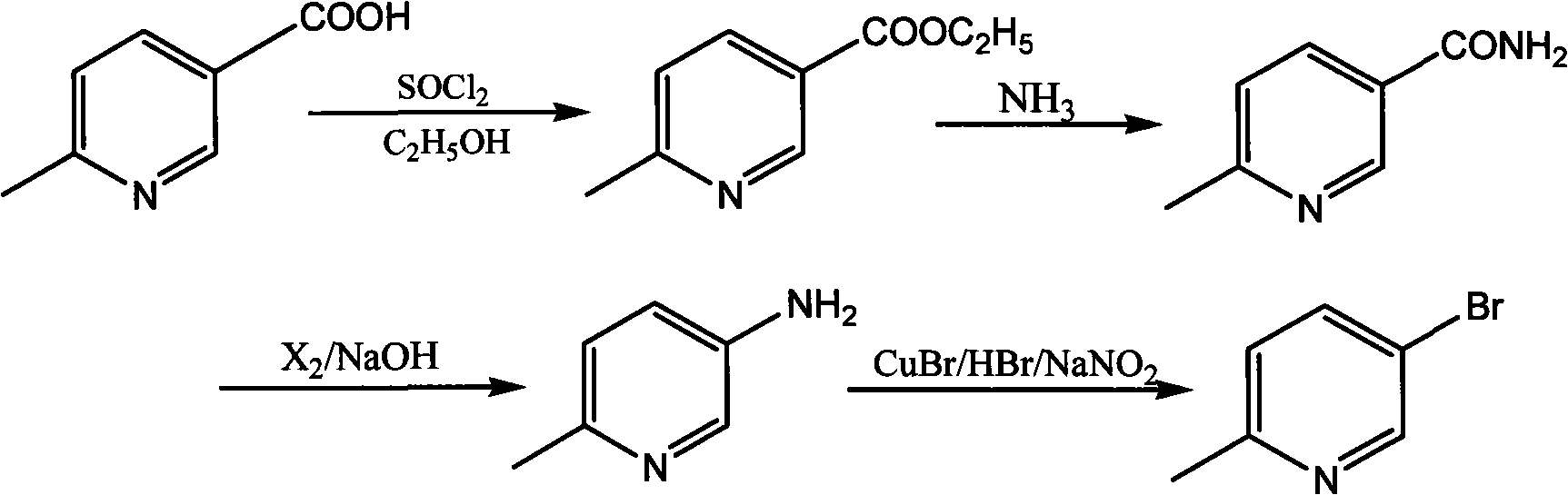
Synthetic method for 5-bromine-2-methylpyridine
A synthesis method and aminopyridine technology, applied in directions such as organic chemistry, can solve problems such as increased cost, wasted time, unsuitable for large-scale industrial production, etc., and achieve the effects of reducing waste, reducing load, and avoiding the generation of 3-position by-products.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] In a 500mL three-necked flask equipped with a stirrer and a thermometer, add 320mL of absolute ethanol, and then add 25.6g (0.19mol) 6-methyl-3-picolinic acid, 20mLSOCl 2 , Install a reflux condenser, control the temperature of the oil bath at 50-60°C, and follow the reaction until the reaction is complete. Spin the solvent to dryness, pour the solid into 200mL saturated NaCO 3 In solution, use CH 2 Cl 2 Extract (100mL*2), combine the organic layers, dry with 20g anhydrous sodium sulfate, filter, spin-dry 28g of brown liquid ethyl 6-methyl-3-picolinate as the solvent, yield 91%, bp 116~117 ℃.
[0032] In a 250mL single-neck flask equipped with magnetic stirring, add 176mL ammonia (concentration 25%), 22g (0.13mol) ethyl 6-methyl-3-pyridinecarboxylate, put into the single-neck flask and stir at room temperature 10~15℃ After 6-7 hours, put in the refrigerator overnight, let the white crystals be fully analyzed, filtered, and washed with water (50 mL*2) to obtain 16.7 g of whi...
Embodiment 2
[0036] In a 500mL three-necked flask equipped with a stirrer and a thermometer, add 300mL of absolute ethanol, and then add 25.6g (0.19mol) 6-methyl-3-picolinic acid, 20mL of concentrated hydrochloric acid, and install a reflux condenser. The temperature of the bath is controlled at 70-80°C, and the reaction is followed until the reaction is complete. Spin the solvent to dryness, pour the solid into 200mL saturated NaCO 3 In solution, use CH 2 Cl 2 Extract (100mL*2), combine the organic layers, dry with 20g anhydrous sodium sulfate, filter, spin-dry 25g of brown liquid ethyl 6-methyl-3-pyridinecarboxylate as solvent, yield 81%, bp 116~117 ℃.
[0037] In a 250mL single-necked flask equipped with magnetic stirring, add 100mL ammonia (concentration 28%), 22g (0.13mol) ethyl 6-methyl-3-pyridinecarboxylate, put into the single-necked flask and stir at room temperature 0~10℃ Place in the refrigerator overnight for 4 to 5 hours, allow the white crystals to be fully analyzed, filter, and ...
Embodiment 3
[0041] In a 500mL three-necked flask equipped with a stirrer and a thermometer, add 350mL of absolute ethanol, and then add 25.6g (0.19mol) 6-methyl-3-picolinic acid, 15mLH 2 SO 4 , Install a reflux condenser, control the temperature of the oil bath at 70-80°C, and follow the reaction until the reaction is complete. Spin the solvent to dryness, pour the solid into 200mL saturated NaCO 3 In solution, use CH 2 Cl 2 Extract (100mL*2), combine the organic layers, dry with 20g of anhydrous sodium sulfate, filter, spin off 26g of brown liquid ethyl 6-methyl-3-picolinate as the solvent, yield 84.5%, bp 116~117 ℃.
[0042] In a 250mL single-neck flask equipped with magnetic stirring, add 200mL ammonia (concentration 25%), 22g (0.13mol) ethyl 6-methyl-3-pyridinecarboxylate, put into the single-neck flask and stir at room temperature 15~25℃ After 7-8 hours, put in the refrigerator overnight, allow the white crystals to be fully analyzed, filtered and washed with water (50mL*2) to obtain 17g...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com