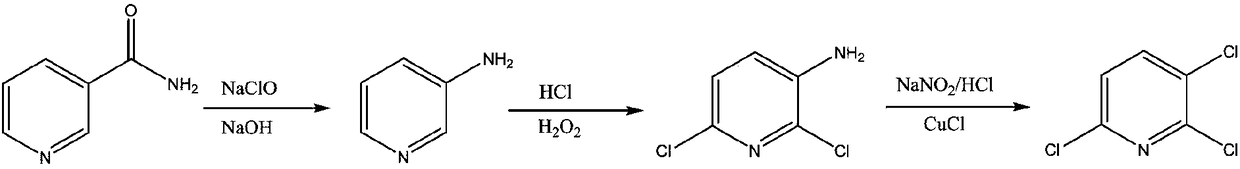
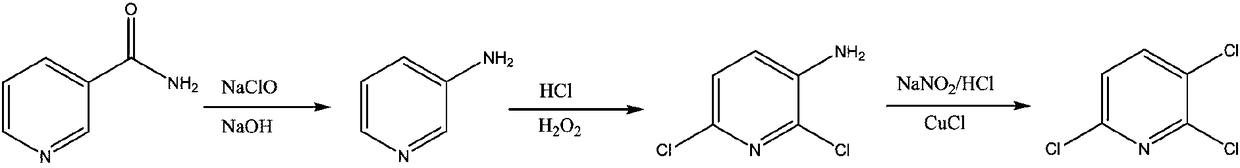
Process study for synthesizing 2,3,6-trichloropyridine from nicotinamide
A trichloropyridine and process research technology, which is applied in the field of nicotinamide synthesis of 2,3,6-trichloropyridine, can solve the problems of large amount of waste water, unavailable raw materials and high cost, so as to reduce the amount of waste water and promote two-phase Full response, cheap effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
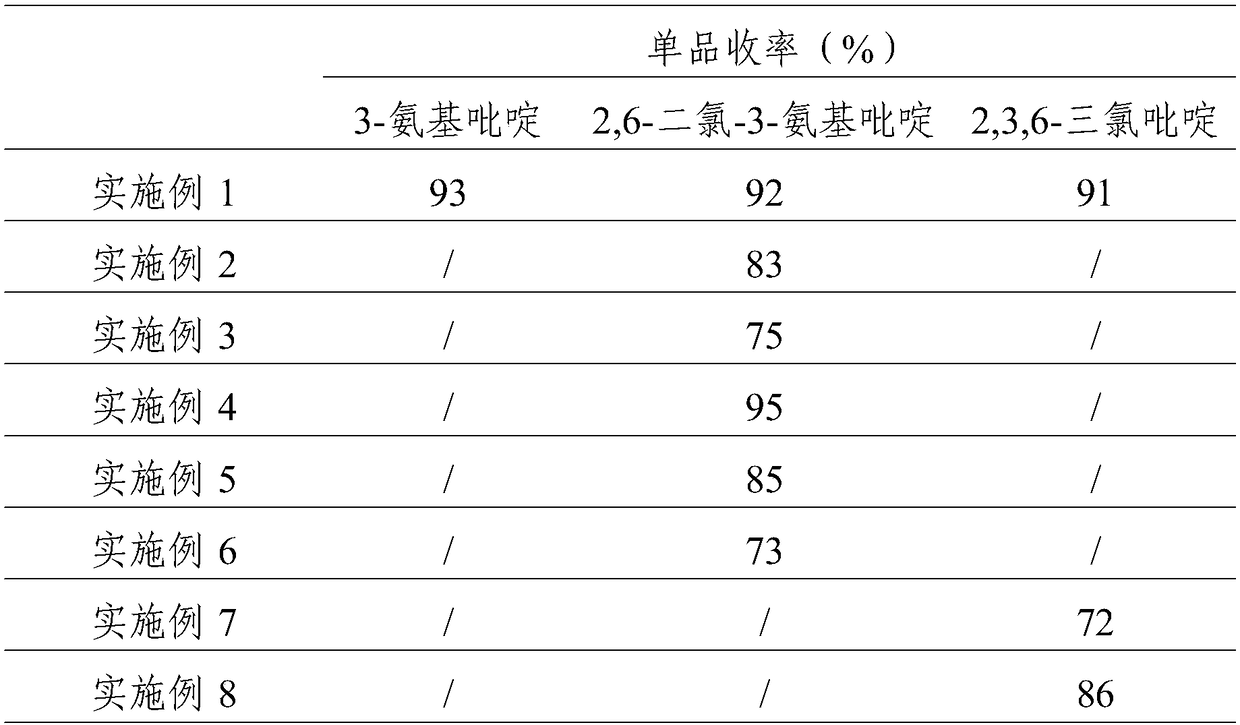
Embodiment 1
[0033] (1) Synthesis of 3-aminopyridine: At room temperature, add 500g of 10% sodium hydroxide solution to the reactor, lower the temperature below 10°C, add 61g of nicotinamide, stir to form a white turbid liquid, add dropwise 343g of 12.5 % sodium hypochlorite solution, control the dropping temperature to be less than 10°C, the reaction solution becomes clear and light yellow-green after dropping, rise to room temperature and stir for 0.5h, continue to heat up to about 90°C, keep warm for 2-3h, and detect nicotinamide by liquid chromatography The content is less than 0.1%. After the reaction is over, cool down to below 10°C, slowly add solid sodium hydroxide until the solution becomes white and turbid, then add ethyl acetate for extraction, and concentrate the organic phase to obtain 43.8g of light yellow solid 3-aminopyridine with a content of more than 95%, crude yield 93%.
[0034] (2) Synthesis of 6-dichloro-3-aminopyridine: add 37% hydrochloric acid 551.6g and Lewis aci...
Embodiment 2
[0037] (1) Synthesis of 2,6-dichloro-3-aminopyridine:
[0038]551.6 g of 37% hydrochloric acid was added into the reactor, and 43.8 g of self-made 3-aminopyridine was added slowly, the solution was raised to room temperature, and the solution turned pale yellow. Cool down to below 15°C, and start to add 74g of 30% hydrogen peroxide solution dropwise. After the dropwise addition, raise the temperature to 35-45°C, keep the temperature for 3-5 hours, and control the 3-aminopyridine in the liquid phase to less than 0.1%, and the reaction ends. Cool down to about 10°C, add saturated sodium metabisulfite solution to neutralize excess hydrogen peroxide until the starch potassium iodide test paper has no color change. Add 50% sodium hydroxide to adjust the pH to 3, add toluene to extract three times, combine the organic phases, then add 30% industrial hydrochloric acid to back-extract the by-product 2-chloro-3-aminopyridine in the organic phase, and concentrate the organic phase under...
Embodiment 3
[0040] (1) Synthesis of 2,3,6-trichloropyridine: Add 69.2g of homemade 2,6-dichloro-3-aminopyridine and 207g of 30% industrial hydrochloric acid, lower the temperature to below 0°C, and start to add 117.2g of 30 % sodium nitrite solution, after the dropwise addition, a diazonium salt solution was prepared, which was kept warm at about 0°C for later use. At room temperature, add 8.4g of cuprous chloride and 207g of 30% industrial hydrochloric acid to the reactor, and start to drop the diazonium salt solution at about 0°C under the protection of nitrogen, and gradually raise the temperature to 60-70°C after the addition, and react for about 2 hours. After the reaction was completed, dichloromethane was added to extract the reaction solution, and the organic phase was concentrated to obtain crude 2,3,6-trichloropyridine. Then add toluene and petroleum ether for recrystallization to obtain 58.1 g of white crystals with a content of 98% and a crude yield of 75%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com