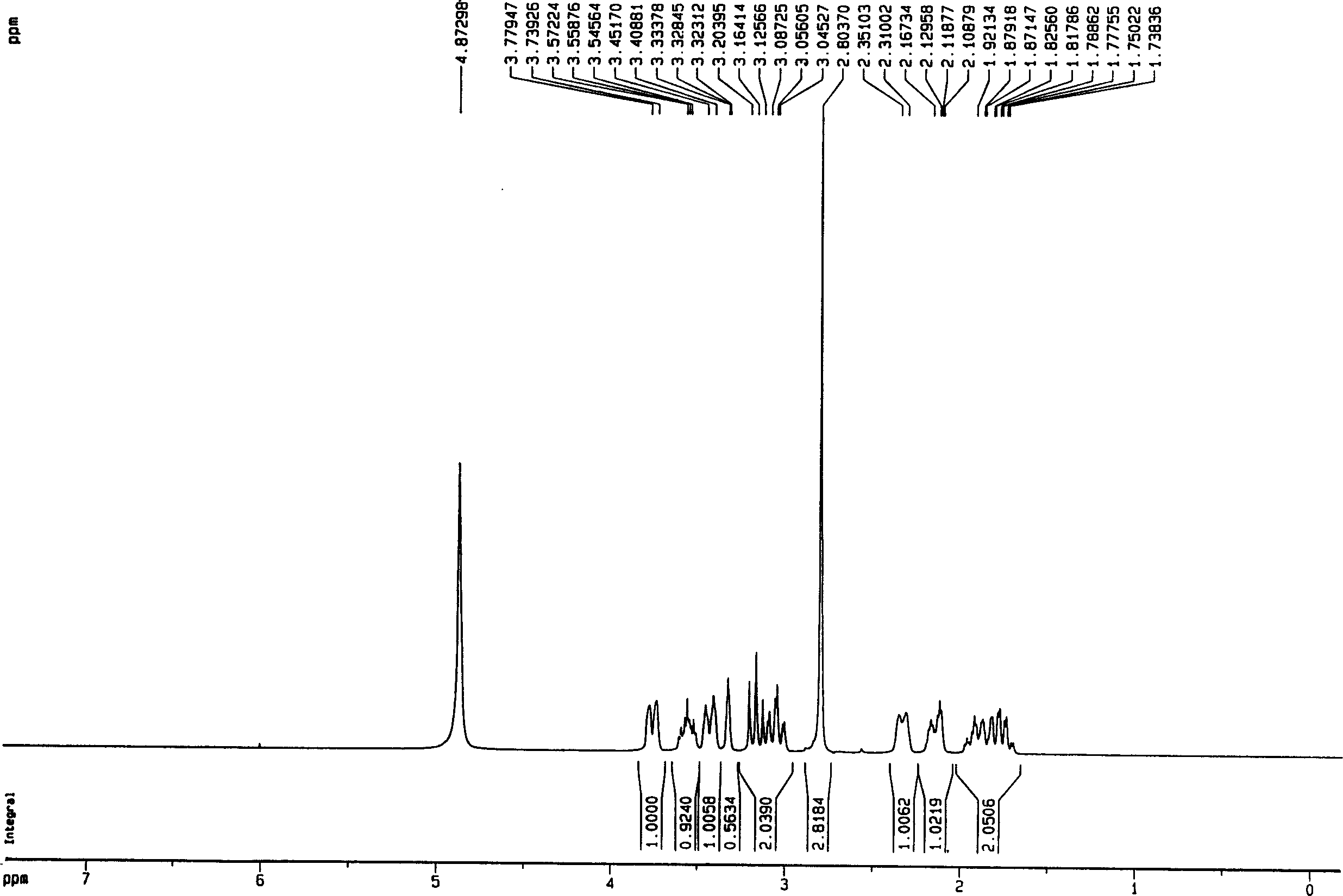
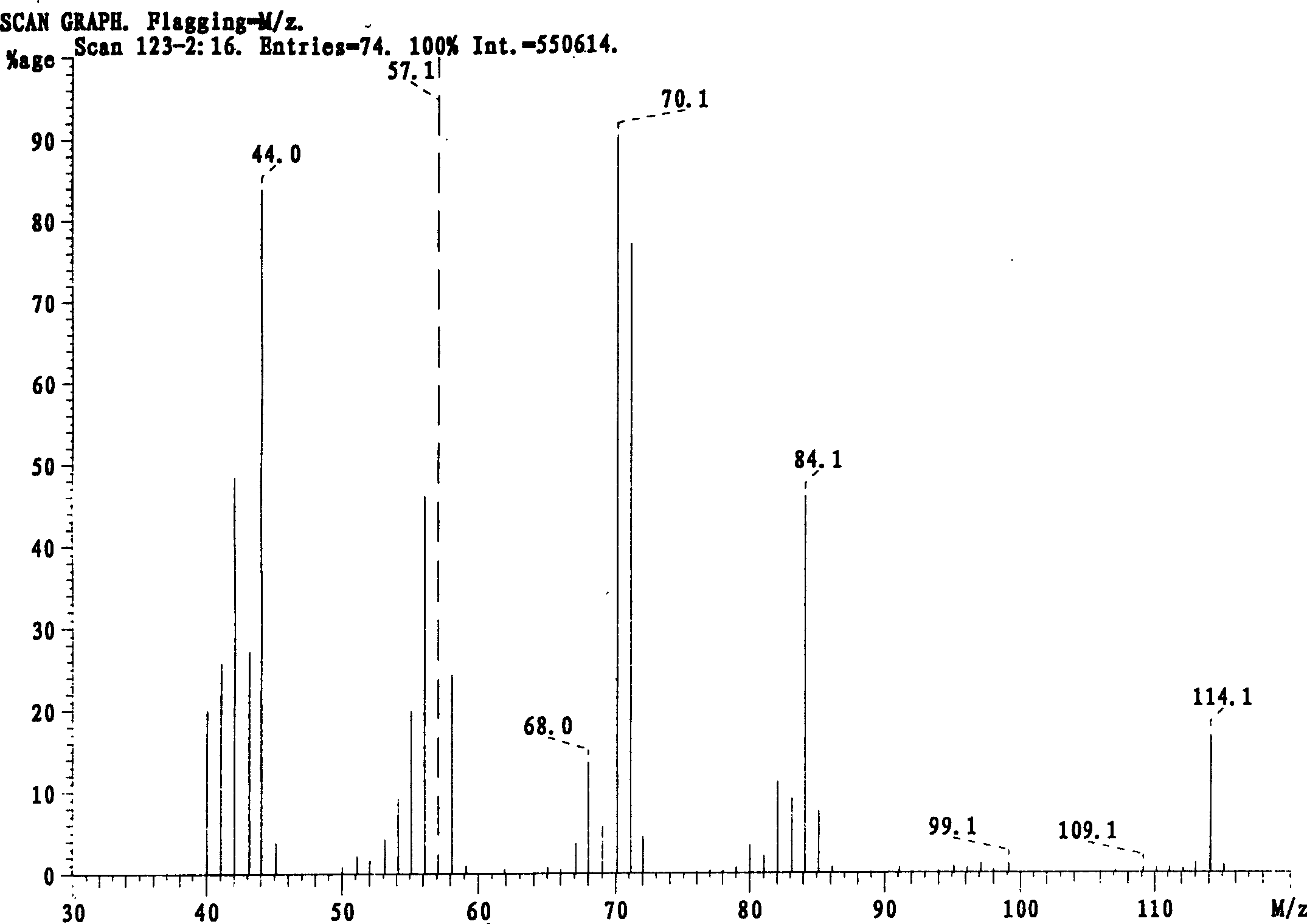
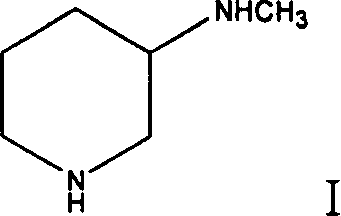
Method for preparing 3-methylamino piperidine and its salt
A technology of aminopyridine and alkyl, applied in the field of preparation of 3-methylaminopiperidine and its salts, can solve the problem of high cost, achieve the effects of reducing production cost, simplifying process operation, and shortening reaction steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] In a 250ml three-necked flask, add 18.8g (0.2mol) of 3-aminopyridine and 220ml (2mol) of trimethyl orthoformate, and stir and reflux for 10h. After the reaction, the solvent was distilled off under reduced pressure. The residue was subjected to fractional distillation under reduced pressure, and the 58-60°C / 100Pa fraction was collected to obtain the compound of formula II (R 1 It is methyl) 20.4g (0.15mol) of colorless liquid, yield 75%. The TLC method followed the reaction process, the developer was ethyl acetate, observed under 254nm fluorescent lamp, the product spot R f = 0.8.
Embodiment 2
[0032] In a 250ml three-neck flask, add 18.8g (0.2mol) of 3-aminopyridine and 160ml (1.22mol) of triethyl orthoformate, and stir and reflux for 5h. After the reaction, the solvent was distilled off under reduced pressure. The residue was subjected to fractional distillation under reduced pressure, and the fraction at 60-62°C / 100Pa was collected to obtain the compound of formula II (R 1 It is ethyl) 25.5g (0.17mol) of colorless liquid, yield 85%. The TLC method followed the reaction process, the developer was ethyl acetate, observed under 254nm fluorescent lamp, the product spot R t = 0.8.
Embodiment 3
[0034] In a 250ml three-necked flask, add 18.8g (0.2mol) of 3-aminopyridine and 40ml (0.3mol) of triethyl orthoformate, and stir and reflux for 6h. After the reaction, the solvent was distilled off under reduced pressure. The residue was subjected to fractional distillation under reduced pressure, and the fraction at 60-62°C / 100Pa was collected to obtain the compound of formula II (R 1 (Ethyl) 18g of colorless liquid, yield 60%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com