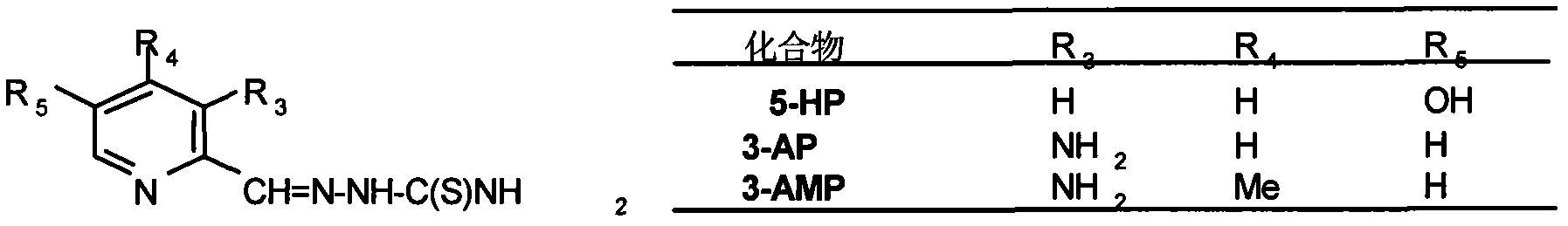
Use of composition containing 3-aminopyridine-2-formaldehyde thiosemicarbazone in treatment of cancers
A technology of thiosemicarbazone and aminopyridine, which is applied in the directions of medical preparations, pharmaceutical formulations, antitumor drugs, etc. containing active ingredients, can solve problems such as increasing the incorporation of gemcitabine DNA
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
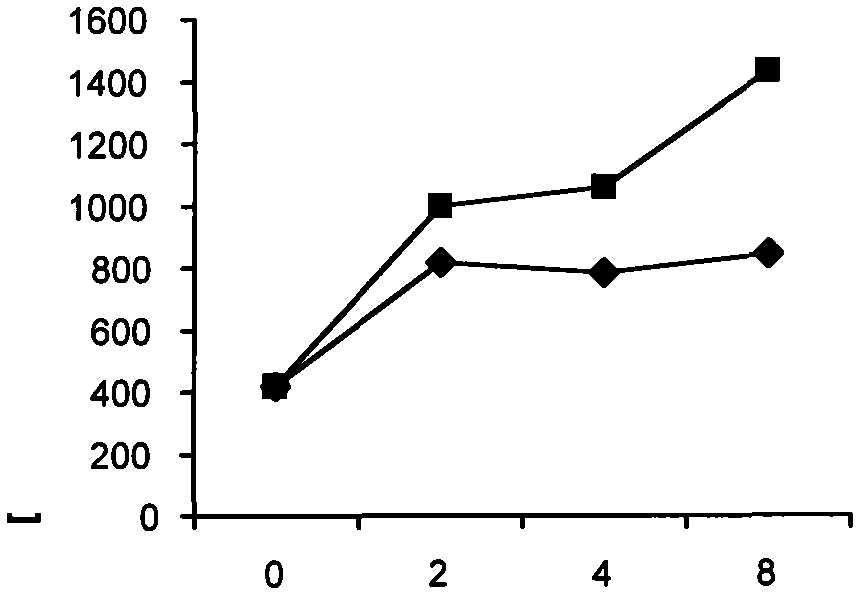
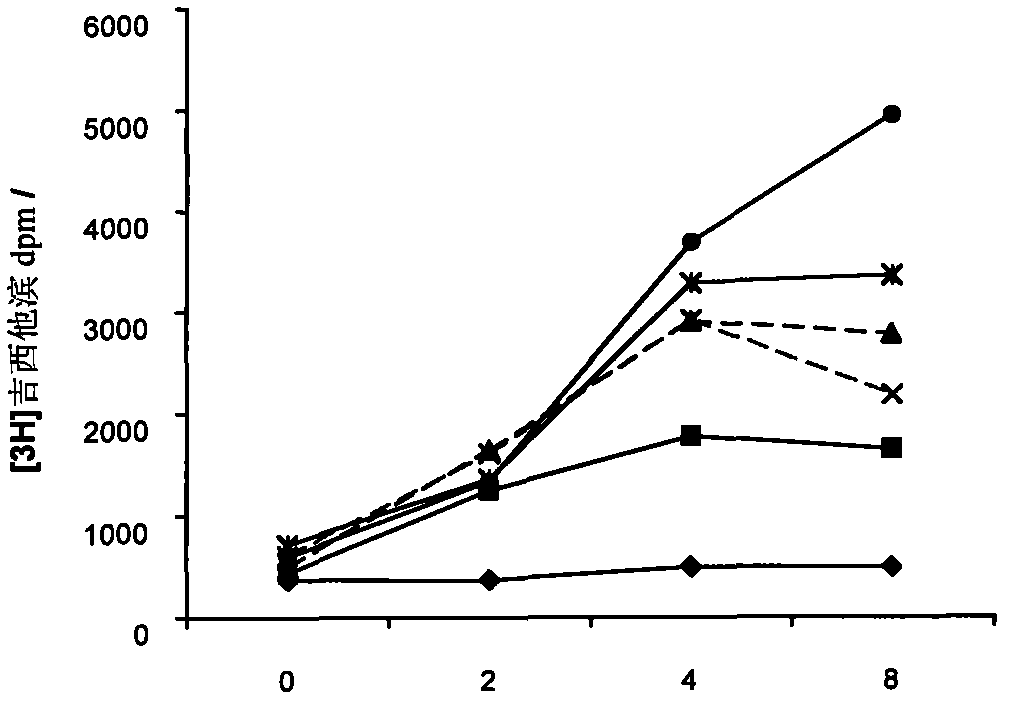
[0045] Improved effect of gemcitabine and cytarabine on 3AP
[0046] 3AP inhibits RR activity leading to depletion of nucleotide pools essential for DNA replication and repair. Therefore, nucleoside analogs capable of incorporation into DNA and causing cytotoxicity after 3AP treatment should be better able to compete with natural nucleotides. This concept was assessed by detecting the incorporation of radiolabeled gemcitabine or cytarabine (ara-C) into the DNA of KB human pharyngeal carcinoma cells at various times after 3AP treatment (Figures 2 and 3) (Wright JA, et al Biochem.Cell Biol. 68:1364-1990).
[0047] In this study, after cells were treated with various concentrations of 3AP for a certain period of time, the cells were washed twice to remove extracellular 3AP. Add 0.3 μM immediately after 3AP exposure [ 3 H]-gemcitabine or [ 3 H]-cytarabine, incubated at 37°C for 30 minutes. DNA was extracted and incorporated label was quantified by scintillation spectrometry. ...
Embodiment 2
[0054] Production of 3AP for intravenous administration
[0055] The active ingredient, 3AP, was added to polyethylene glycol-300 (PEG) (approximately 7.1 mg / mL of 3AP in PEG) and homogenized to reduce particle size. Then ethanol containing citric acid (20 mg / mL) and ascorbic acid (3.3 mg / mL) was added and mixed until a clear solution was obtained (ethanol:PEG=3:7, v / v). Pneumatic mixing shafts are also used when extended mixing is required to dissolve drugs. The solution was flushed with nitrogen to prevent oxidative degradation of 3AP during mixing. The solution was filtered first through a 0.45 μm membrane filter and then through 2 sterile 0.22 μm membrane filters used in succession. Filter integrity checks were performed using bubble point measurements before and after filtration. The filling equipment and 20mm rubber stoppers were sterilized by autoclaving at 122.5°C for a minimum of 40 minutes. The 10-mL Class I amber vials were washed and depyrogenated for a minimum...
Embodiment 3
[0057] Protects 3AP from degradation induced by light exposure
[0058] Two bottles of 3AP injection were exposed to light (1200J) for 1.5 hours at room temperature. One in a clear glass vial and the other in an amber glass vial sealed with a butyl synthetic rubber stopper and an aluminum crimp. Samples were analyzed by HPLC in duplicate at each time point.
[0059] The stability of 3AP after light exposure was analyzed by HPLC with ultraviolet (UV) detector. An Agilent technologies 1100 series HPLC system comprising a quad gradient pump, autosampler, column heater and multiwavelength or photodiode array detector was used. The sample was analyzed with a Supelco LC-18-T column (5 μm, 250x6.0mm), and the mobile phase consisted of 25% MeOH / 75% 50mM KH 2 PO 4 and 1 mM EDTA. Data acquisition and processing was performed using Agilent ChemStation software. Table 4 summarizes the results of light exposure of 3AP stored in clear or amber vials. Apparently, the amber amber vial ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com