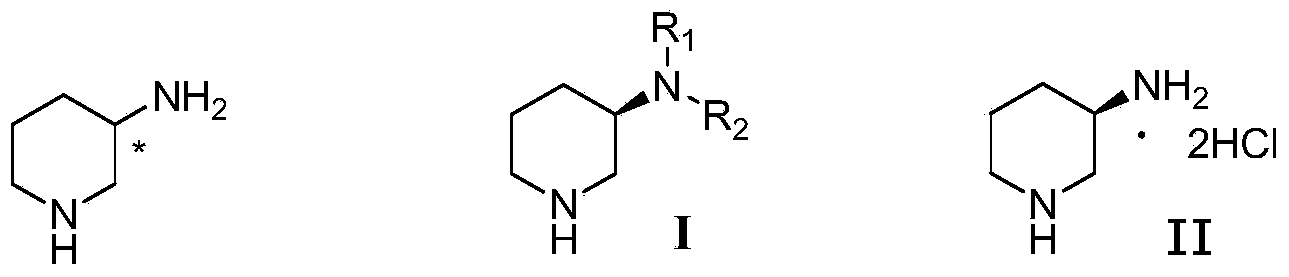
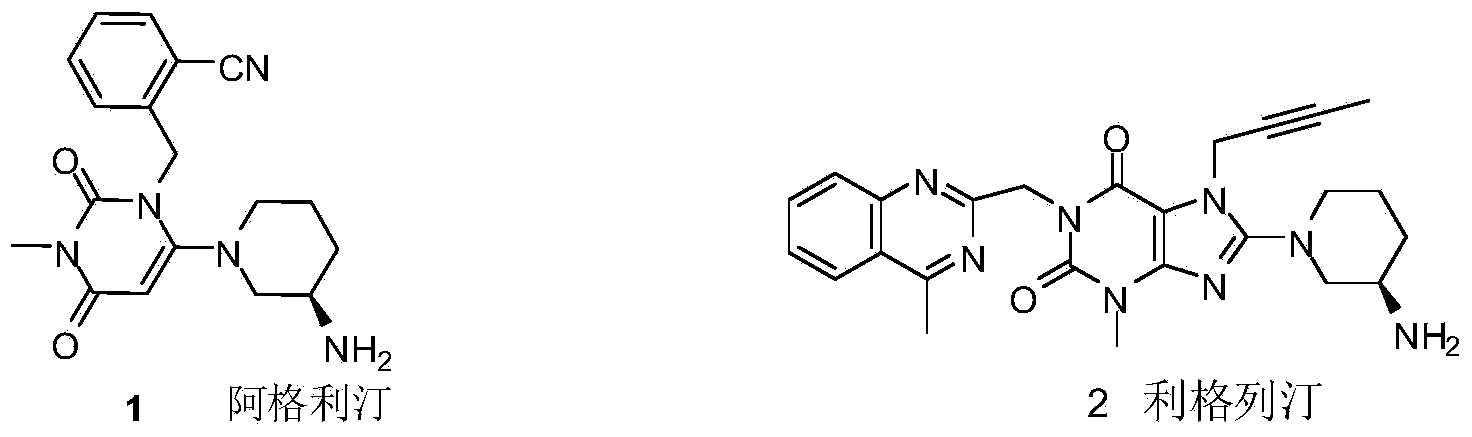
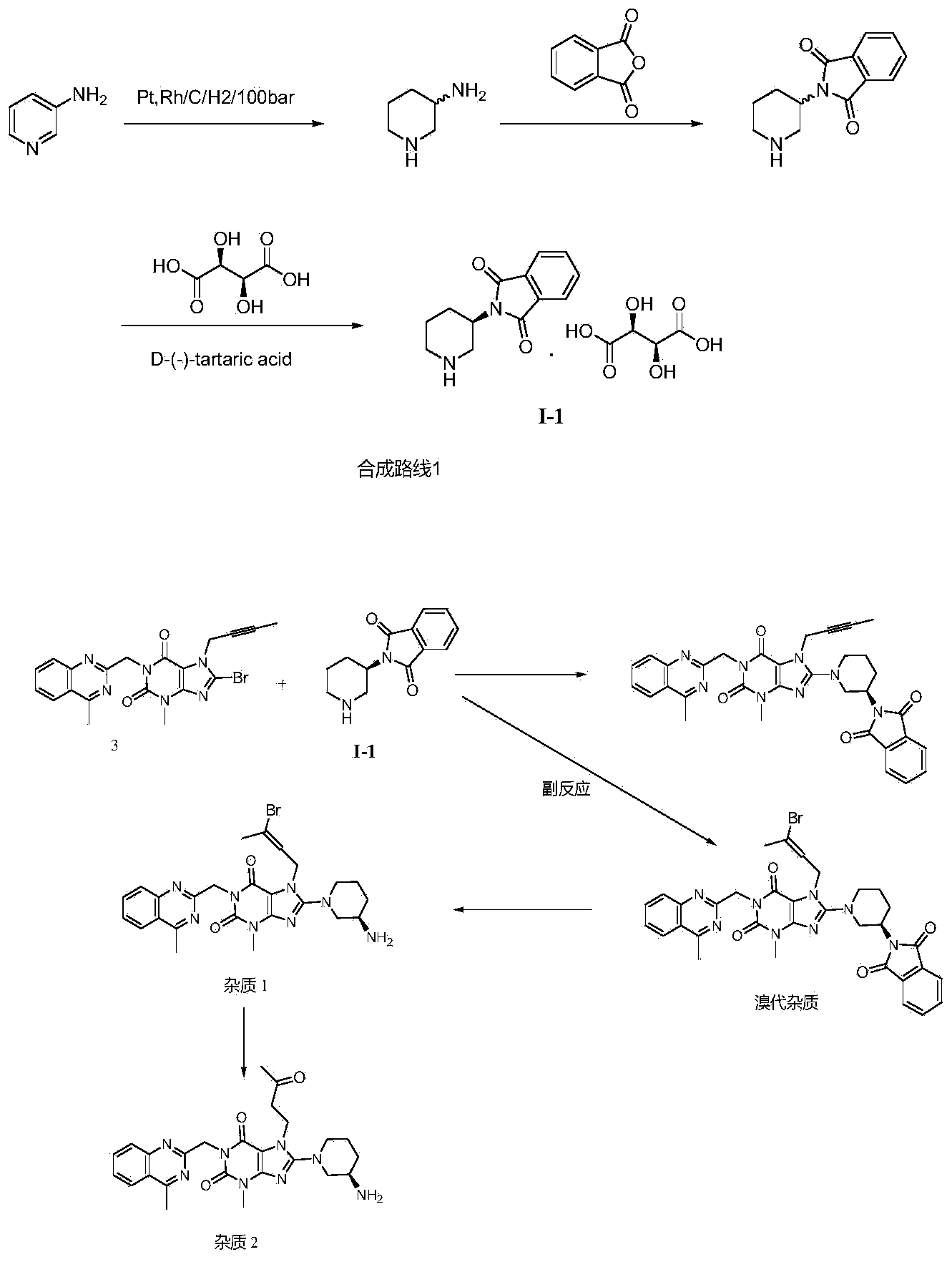
Compound I and (R)-3-aminopiperidine hydrochloride II, preparation method and application in Linagliptin synthesis
A compound, aminopyridine technology, applied to compound I and (R)-3-aminopiperidine hydrochloride II, its preparation and application field in linagliptin synthesis, can solve the problem that the product enters the water phase, 3-Aminopiperidine is expensive in the market and difficult to realize industrialized production and other problems
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0080] Example 1: 2,2,2-trifluoro-N-(3-pyridyl)acetamide
[0081]
[0082] 3-Aminopyridine (60g, 0.637mol), tetrahydrofuran (THF, 300ml) were added to the reaction vessel. Stir and cool down to 0°C. Trifluoroacetic anhydride (160 g, 0.764 mol) was added dropwise to the reaction mixture. React at room temperature for 3 hours. Saturated aqueous sodium bicarbonate solution (100 ml) was added dropwise to the reaction solution, followed by stirring for 0.5 hours. The organic layer was collected and concentrated to give 2,2,2-trifluoro-N-(3-pyridyl)acetamide.
[0083] Yield: 118.6 g (98% of theory)
Embodiment 2
[0084] Embodiment 2:: N-(3-pyridyl)benzamide
[0085]
[0086] 3-Aminopyridine (50 g, 0.531 mol), ethyl acetate (EA, 250 ml) and triethylamine (64 g, 0.637 mol) were added to the reaction vessel. Stir and cool down to 0°C. Benzoyl chloride (78 g, 0.557 mol) was added dropwise to the reaction mixture. React at 0-5°C for 5 hours. Filter and collect the filtrate. The filtrate was washed with saturated brine. The organic layer was collected and concentrated to give N-(3-pyridyl)benzamide.
[0087] Yield: 84.2 g (80% of theory)
Embodiment 3
[0088] Example 3: 1-(3-pyridyl)-2,5-pyrrolidinedione
[0089]
[0090] Add 3-aminopyridine (10g, 0.106mol) and toluene (100ml) into the reaction vessel under nitrogen protection, stir to dissolve. Succinic anhydride (15.9 g, 0.159 mol) and triethylamine (21.4 g, 0.211 mol) were added to the reaction flask, and the temperature was raised to reflux for 8 hours to separate the water in the system.
[0091] Stop the heating reaction, cool down to room temperature naturally, then cool down to about 0°C with an ice-water bath and stir for 1 hour. Filter and wash the filter cake three times with toluene (30ml*3). The filter cake was collected and dried to obtain 1-(3-pyridyl)-2,5-pyrrolidinedione.
[0092] Yield: 16.8 g (90% of theory)
[0093] 1 H-NMR (400MHz, CDCl3): δ2.93-2.94 (m, 4H), 7.42-7.46 (m, 1H), 7.69-7.72 (m, 1H), 8.61-8.64 (m, 2H).
PUM
| Property | Measurement | Unit |
|---|---|---|
| optical purity | aaaaa | aaaaa |
| optical purity | aaaaa | aaaaa |
| optical purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com