Method for preparing poly-substituted 1, 5-naphthyridine compound
A compound and multi-substitution technology, applied in organic chemistry and other fields, can solve problems such as lack of flexibility in substitution forms
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
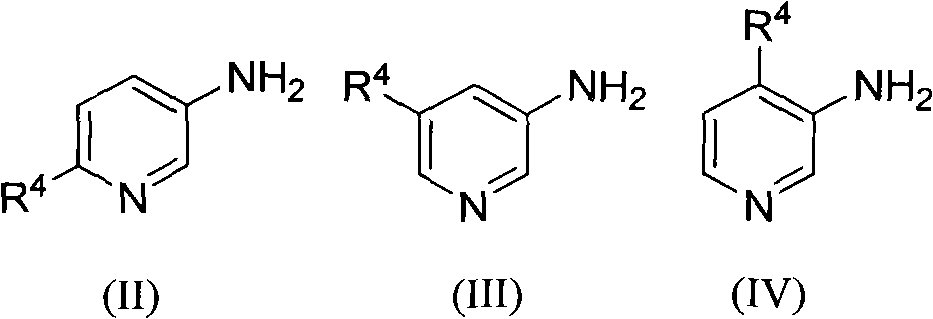
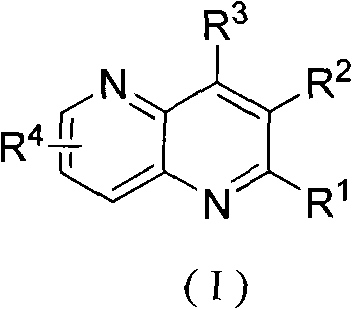
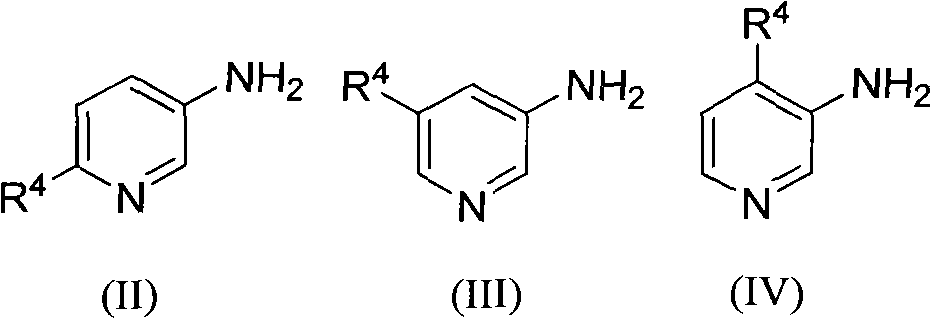
[0023] The preparation of embodiment 1 2-methyl-1,5-naphthyridine (R 1 , R 2 , R 3 for hydrogen, R 4 for 6-methyl)
[0024] Add 0.129 mol of ferrous sulfate heptahydrate, 1.59 mol of 3-aminopyridine and 5.56 mol of 2-butenal to the reactor in sequence, slowly add 2.8 mol of concentrated sulfuric acid, and heat to reflux for 12 hours. Cool to room temperature, neutralize to neutral with sodium hydroxide solution, filter, wash the filter cake with 1L ethyl acetate, extract the filtrate three times, combine the ethyl acetate phases, and rotary evaporate to recover ethyl acetate to obtain a concentrate. The product was distilled under reduced pressure to obtain 112.5 g of 2-methyl-1,5-naphthyridine with a yield of 50%. Colorless crystals, melting point 64°C-65°C. H NMR spectrum ( 1 H NMR, 300MHz, CDCl 3 )δ8.90-8.89 (m, 1H), 8.31-8.25 (m, 2H), 7.60-7.49 (m, 2H), 2.76 (s, 3H).
Embodiment 2
[0025] Embodiment 2 2-1, the preparation of methyl 5-naphthyridine acetate (R 1 , R 2 , R 3 for hydrogen, R 4 6-methylenecarbonyloxymethyl)
[0026] Add 69mmol of 2-methyl 1,5-naphthyridine to the reaction kettle, add redistilled tetrahydrofuran under anhydrous and oxygen-free conditions, raise the temperature to 50°C, add 208mmol of lithium diisopropylamide dropwise, after the dropwise addition Stir at 50°C for 30min, continue to add 76mmol of dimethyl carbonate dropwise, after the dropwise addition, stir at 50°C for 2h, quench the reaction with 20ml of 30% saturated ammonium chloride solution, extract three times with 50ml of ethyl acetate, combine organic phase, concentrated, and distilled under reduced pressure to obtain 7.8 g of methyl 2-1,5-naphthyridine acetate, with a yield of 56%, as colorless crystals, with a melting point of 68°C-70°C. H NMR spectrum ( 1 H NMR, 300MHz, CDCl 3 )δ8.95-8.93 (m, 1H), 8.37-8.33 (m, 2H), 7.66-7.59 (m, 2H), 4.06 (s, 2H), 3.72 (s, 3H)...
Embodiment 3
[0027] Example 3 The preparation of 2-hydroxyl-6-methyl-1,5-naphthyridine (R 1 is methyl, R 2 , R 3 for hydrogen, R 4 for 6-hydroxyl)
[0028] Add 1.8mmol ferrous sulfate heptahydrate, 145mmol 2-hydroxyl-5-aminopyridine and 290mmol 2-butenal in sequence in the reaction kettle, slowly add 110mmol concentrated sulfuric acid, heat and reflux for 2 hours, then cool the reaction system to room temperature, Neutralize to neutral with 88ml of 10% sodium hydroxide solution, filter, wash the filter cake with 150ml of ethyl acetate, extract the filtrate three times, combine the ethyl acetate phases, and remove the ethyl acetate by rotary evaporation to obtain a concentrate. The product was subjected to silica gel column chromatography with ethyl acetate as the eluent to obtain 13.9 g of 2-hydroxy-6-methyl 1,5-naphthyridine, a colorless solid with a yield of 60%. H NMR spectrum ( 1 H NMR, 300MHz, CDCl 3)δ8.04-8.01 (dd, 1H), 7.67 (d, 1H), 7.36-7.26 (d, 1H), 6.92-6.89 (d, 1H), 2.67-2...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com