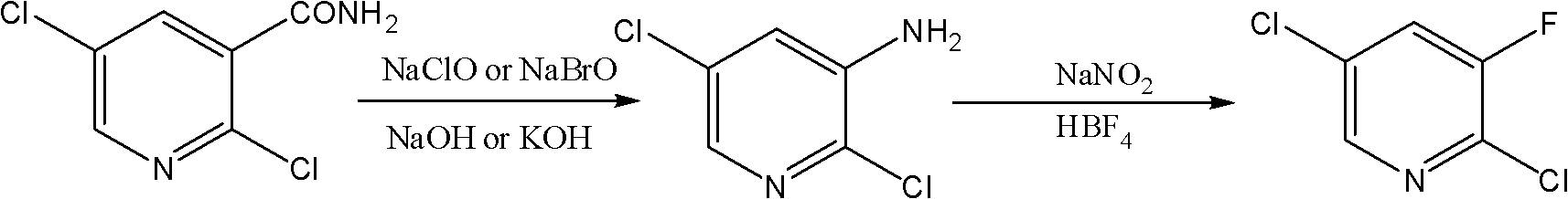
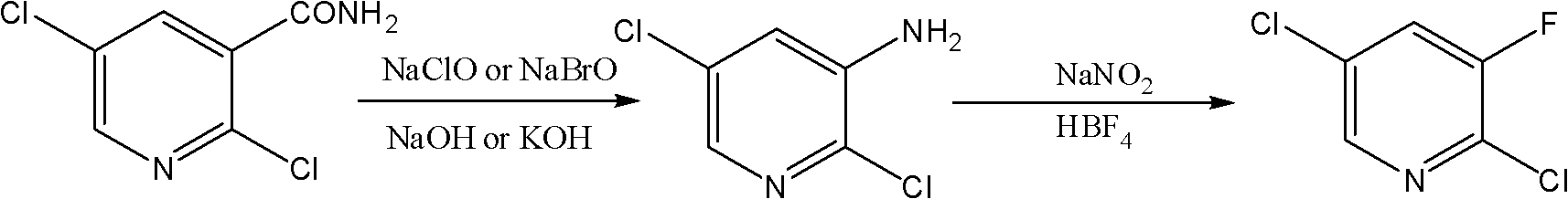
Novel method for synthesizing 2,5-dichloro-3-fluoropyridine
A technology of fluoropyridine and a new method, which is applied in the field of synthesis 2, can solve the problems of low yield and high cost of the target product, and achieve the effects of easy control, mild reaction process and simple reaction steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] Add 100ml of water, 12.4g of sodium hypochlorite, 25g of sodium hydroxide into the four-necked reaction flask, cool down to -5°C, add 20g of 2,5-dichloronicotinamide, keep it warm for 2 hours, and then raise the temperature to 65°C and stir for 5 hours. Hours, cooling down after the reaction, the precipitated solid was 2,5-dichloro-3-aminopyridine, quantitative analysis showed 14g of 2,5-dichloro-3-aminopyridine with a purity of 92.7%. Add 14 g of the obtained 2,5-dichloro-3-aminopyridine to 74.5 g of 40 wt% fluoboric acid, cool to -5°C, add dropwise 35 ml of water containing 7.1 g of sodium nitrite, and keep -5 ℃ for 4 hours, after the reaction was completed, the temperature was raised to 25 ℃ and stirred for 10 hours. After the reaction, a white solid was obtained, which was the fluoroborate of 2,5-dichloro-3-fluoropyridine, and the obtained solid was filtered Heating to 60-70°C in petroleum ether, the white solid salt decomposes to obtain the target product 2,5-dichl...
Embodiment 2
[0022] The sodium hydroxide used in the reaction process is changed into potassium hydroxide, and the molar weight is consistent, all the other reaction conditions are identical with embodiment 1, finally obtain target product 2,5-dichloro-3-fluoropyridine, content is 93.8%, with 2 , 5-dichloronicotinamide calculation, its yield is 67.2%
Embodiment 3
[0024] During the reaction, except that the sodium hydroxide added was 18g, the rest of the reaction conditions were the same as in Example 1, and finally 2,5-dichloro-3-fluoropyridine with a content of 92% was obtained, calculated as 2,5-dichloronicotinamide , whose yield is 65.3%
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com