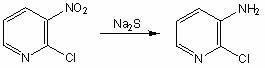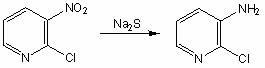Preparation method of 2-chloro-3-aminopyridine
A technology of aminopyridine and nitropyridine, which is applied in the field of preparation of 2-chloro-3-aminopyridine, can solve the problems of difficult refining and decolorization, high price, and low product yield, so as to achieve cheap and easy-to-obtain raw materials and reduce production The effect of high cost and product yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0015] In a four-neck flask equipped with a stirrer, reflux condenser, dropping funnel and thermometer, add 1.0L of water, 158.5g (1.0mol) of 2-chloro-3-nitropyridine, and 360g (6.7mol) of ammonium chloride , 60mL of concentrated ammonia water, heated to 80°C, 1250mL (3.5mol) of 20% sodium sulfide solution was added dropwise, the temperature was kept between 75-80°C during the dropwise addition process, and the addition was completed within 1h. After adding the sodium sulfide solution, continue the heat preservation reaction for 15 minutes, filter while it is hot, and cool the filtrate to precipitate a solid. Add 5% dilute hydrochloric acid to the solid until the solid is no longer dissolved. After filtration, the filtrate was adjusted to pH 9 with 15% NaOH solution, and crystals were precipitated. Suction filtration, washing with water, and vacuum drying yielded 110.6 g of 2-chloro-3-aminopyridine, with a yield of 86.1% and a content of 98.7% (results obtained by liquid chro...
Embodiment 2
[0017] In a four-neck flask equipped with a stirrer, reflux condenser, dropping funnel and thermometer, add 1.0L of water, 158.5g (1.0mol) of 2-chloro-3-nitropyridine, and 268.0g (5mol) of ammonium chloride , 60mL of concentrated ammonia water, heated to 75°C, 1250mL (3.5mol) of 20% sodium sulfide solution was added dropwise, the temperature was kept between 70-80°C during the dropwise addition process, and the addition was completed within 1h. After adding the sodium sulfide solution, continue the heat preservation reaction for 15 minutes, filter while it is hot, and cool the filtrate to precipitate a solid. Add 5% dilute hydrochloric acid to the solid until the solid is no longer dissolved. After filtering, the filtrate was adjusted to pH 10 with 15% NaOH solution, and crystals were precipitated. Suction filtration, washing with water, and vacuum drying gave 95.4 g of 2-chloro-3-aminopyridine, yield 74.2%, content 96.2%, m.p.79-81°C. The NMR data of the product obtained: ...
Embodiment 3
[0019] In a four-neck flask equipped with a stirrer, reflux condenser, dropping funnel and thermometer, add 1.0L of water, 158.5g (1.0mol) of 2-chloro-3-nitropyridine, 360.0g (6.7mol) of ammonium chloride ), concentrated ammonia water 60mL, heated to 80°C, and 1000mL (2.8mol) of 20% sodium sulfide solution was added dropwise, keeping the temperature between 75-80°C during the dropwise addition process, and the addition was completed within 1h. After adding the sodium sulfide solution, continue the heat preservation reaction for 15 minutes, filter while it is hot, and cool the filtrate to precipitate a solid. Add 5% dilute hydrochloric acid to the solid until the solid is no longer dissolved. Filter and adjust the pH value of the filtrate to 9-10 with 15% NaOH solution to precipitate crystals. Suction filtration, washing with water, and vacuum drying gave 102.2 g of 2-chloro-3-aminopyridine, yield 79.5%, content 97.6%, m.p.79-81°C. The NMR data of the product obtained: 1 HNM...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com