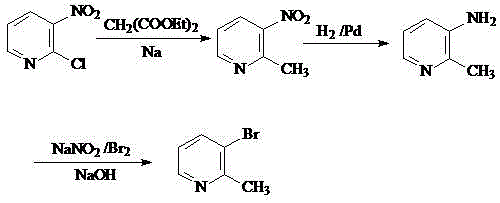
Preparation method of 2-methyl-3-bromopyridine
A technology of bromopyridine and nitropyridine, which is applied in the field of preparation of 2-methyl-3-bromopyridine, can solve the problems of many by-products and low yield, and achieve low production cost, high yield, and cheap raw material prices Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019] (1) Preparation of 2-methyl-3-nitropyridine: Heat a mixture of diethyl malonate (80ml, 0.5mol) and sodium (2.53g, 0.11mol) in an oil bath to 90°C, stir for 1h, After heating up to 120°C and stirring for 45 min, it was cooled to room temperature. A toluene solution of 2-chloro-3-nitropyridine (15.6 g, 0.1 mol) was added dropwise. After the addition was complete, the reaction solution was heated to 110° C. for 1.5 h, cooled to room temperature and stirred for 15 h. The solvent was evaporated under reduced pressure, 6N hydrochloric acid (100ml) was added, the temperature was raised to reflux for 3.5h and then cooled to room temperature. Adjust the pH to alkaline with saturated sodium carbonate solution, extract with ethyl acetate, combine the base phases, dry over anhydrous sodium sulfate, and concentrate by suction filtration to obtain 2-methyl-3-nitropyridine with a molar yield of 92%. .
[0020] (2) Preparation of 2-methyl-3-aminopyridine: Dissolve 2-methyl-3-nitropyr...
Embodiment 2
[0023] (1) Preparation of 2-methyl-3-nitropyridine: Heat a mixture of diethyl malonate (80ml, 0.5mol) and sodium (2.76g, 0.12mol) in an oil bath to 90°C, stir for 1h, After heating up to 120°C and stirring for 45 min, it was cooled to room temperature. A toluene solution of 2-chloro-3-nitropyridine (15.6 g, 0.1 mol) was added dropwise. After the addition was complete, the reaction solution was heated to 110° C. for 1.5 h, cooled to room temperature and stirred for 15 h. The solvent was evaporated under reduced pressure, 6N hydrochloric acid (100ml) was added, the temperature was raised to reflux for 3.5h and then cooled to room temperature. Adjust the pH to alkaline with saturated sodium carbonate solution, extract with ethyl acetate, combine the base phases, dry over anhydrous sodium sulfate, and concentrate by suction filtration to obtain 2-methyl-3-nitropyridine with a molar yield of 95%. .
[0024] (2) Preparation of 2-methyl-3-aminopyridine: Dissolve 2-methyl-3-nitropyr...
Embodiment 3
[0027] (1) Preparation of 2-methyl-3-nitropyridine: Heat a mixture of diethyl malonate (80ml, 0.5mol) and sodium (2.99g, 0.13mol) in an oil bath to 90°C, stir for 1h, After heating up to 120°C and stirring for 45 min, it was cooled to room temperature. A toluene solution of 2-chloro-3-nitropyridine (15.6 g, 0.1 mol) was added dropwise. After the addition was complete, the reaction solution was heated to 110° C. for 1.5 h, cooled to room temperature and stirred for 15 h. The solvent was evaporated under reduced pressure, 6N hydrochloric acid (100ml) was added, the temperature was raised to reflux for 3.5h and then cooled to room temperature. Adjust the pH to alkaline with saturated sodium carbonate solution, extract with ethyl acetate, combine the base phases, dry over anhydrous sodium sulfate, and concentrate by suction filtration to obtain 2-methyl-3-nitropyridine with a molar yield of 95%. .
[0028] (2) Preparation of 2-methyl-3-aminopyridine: Dissolve 2-methyl-3-nitropyr...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com