Synthetic methods of ceftazidime intermediate and ceftazidime
A ceftazidime and synthetic method technology, applied in the field of drug synthesis, can solve the problems of low product purity and low process yield, and achieve the effects of less three waste discharge, high product purity, and simplified process flow
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
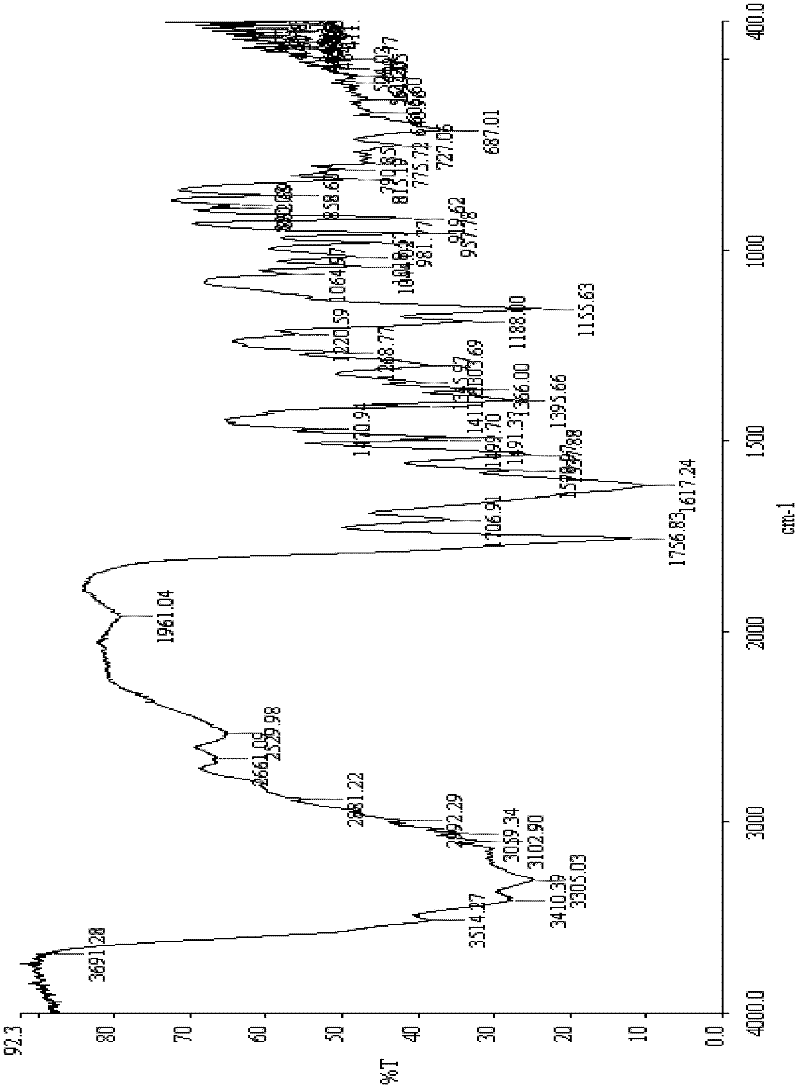
[0044] [embodiment 1] the synthesis of ceftazidime intermediate (formula 1, wherein HX is HCl) monohydrate:
[0045] 30g (0.11mol) of 7-aminocephalosporanic acid, 300ml of dichloromethane, and 35ml (0.16mol) of hexamethyldisilazane were placed in a reaction flask, heated to reflux for 8 hours, and N,N-diethyl Aniline 29ml (0.18mol), trimethylsilyl iodide 32g (0.16mol), react at room temperature for 3hr, add pyridine 18ml (0.22mol) under ice bath, and continue to react for 1hr;
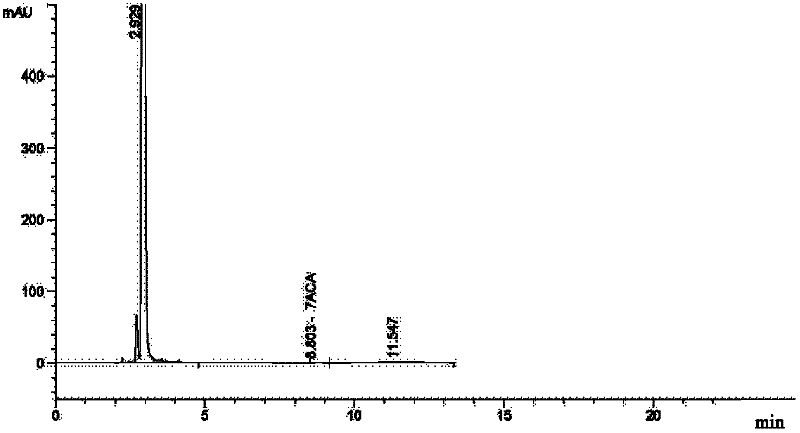
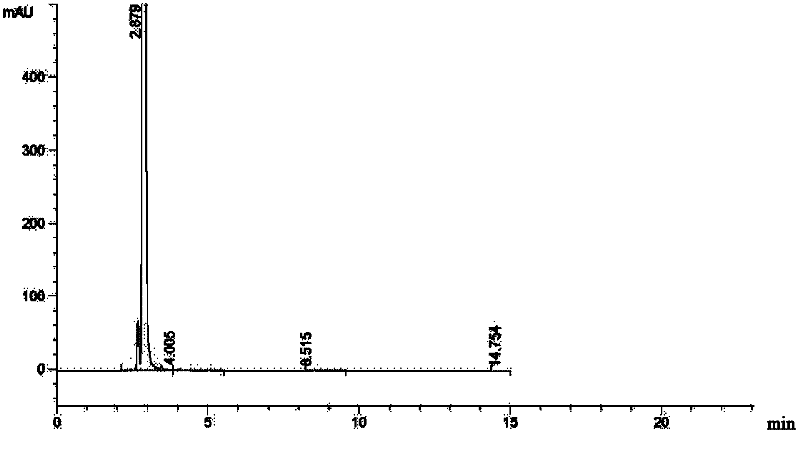
[0046] Add 35ml of methanol dropwise, add 6ml of hydrogen peroxide (0.13mol), 80ml of concentrated hydrochloric acid, and 100ml of water, stir until all the solids are dissolved, let it stand for stratification, add 500ml of acetone to the water phase, add triethylamine to adjust the pH to 3.0, filter, and 100ml of acetone Washing, drying in vacuo to get light yellow solid 34.4g (91%), content 83.5%, purity > 98%, moisture: 5.5%, product detects through potentiometric titration that the chloride ion co...
Embodiment 2
[0047] [embodiment 2] the synthesis of ceftazidime intermediate (formula 1, wherein HX is HCl) monohydrate:
[0048] As described in Example 1, the difference is that the oxidant is replaced by FeCl 3 26g (0.16mol), after extraction, add 500ml of acetone to the water phase, add triethylamine to adjust the pH to 3.0, filter, wash with 100ml of acetone, and dry in vacuo to obtain 34.1g (90%) of a light yellow solid with a content of 83.9% and a purity of >98% , KF: 5.6%, chloride ion content: 10.84%, which is the monohydrochloride monohydrate of the ceftazidime intermediate (Formula 1, wherein HX is HCl).
Embodiment 3
[0049] [embodiment 3] the synthesis of ceftazidime intermediate (formula 1, wherein HX is HCl) monohydrate:
[0050] As described in Example 1, the difference is that the oxidizing agent is replaced by 12ml (0.08mol) of peracetic acid with a mass concentration of 20%. After extraction, 500ml of acetone is added to the water phase, and triethylamine is added to adjust the pH to 3.0, filtered, and washed with 100ml of acetone. , dried in vacuo to give light yellow solid 33.9g (90%), content 84.3%, purity > 98%, KF: 5.2%, chloride ion content: 10.2%, be ceftazidime intermediate (formula 1, wherein HX is HCl) single Hydrochloride monohydrate.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com