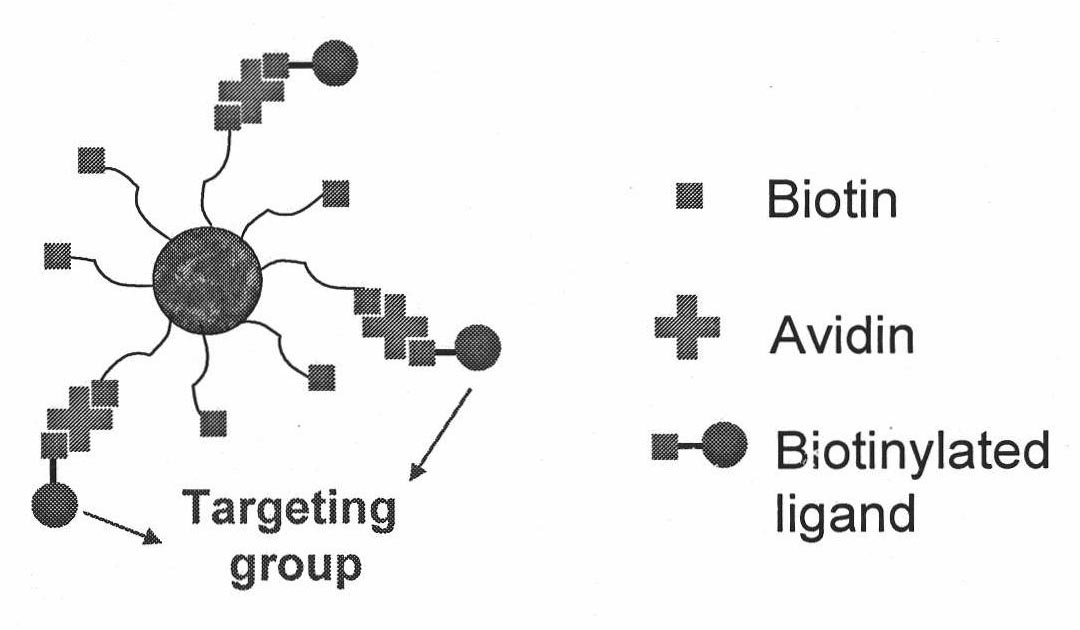
Targeted polymer medicament carrier and preparation method and application thereof
A polymer and targeted technology, applied in the field of biomedicine
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
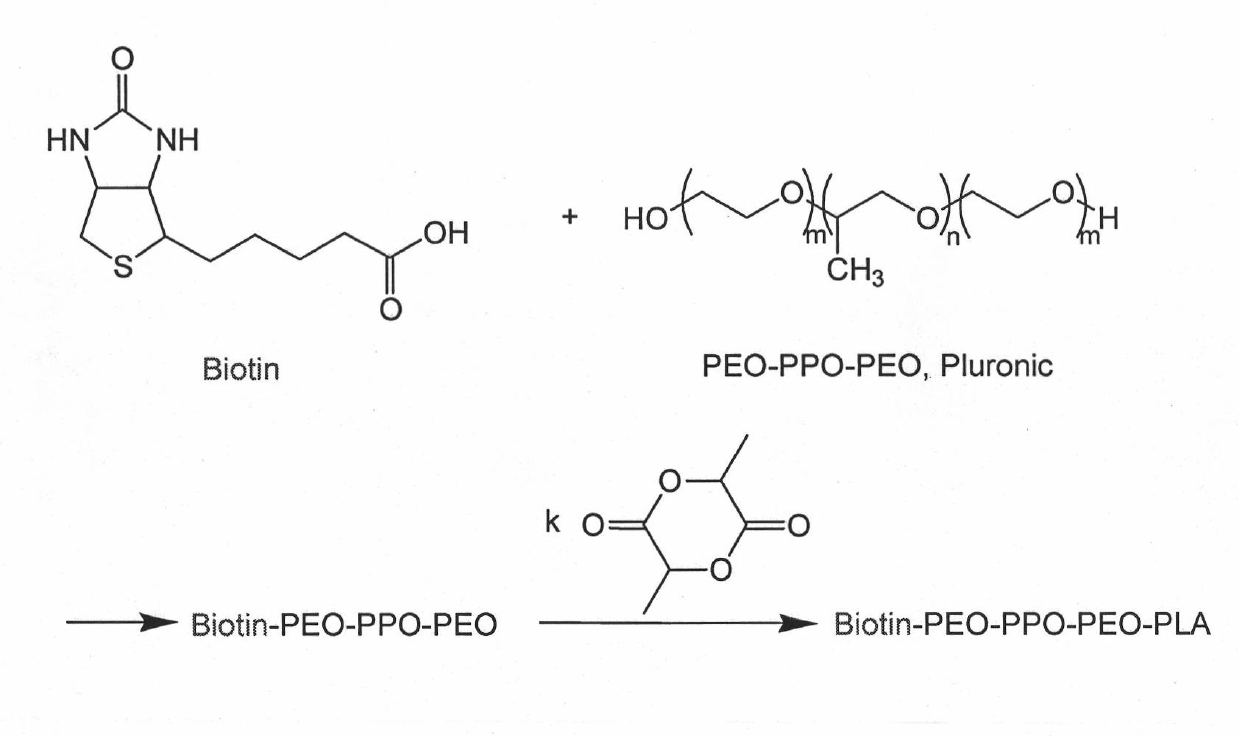
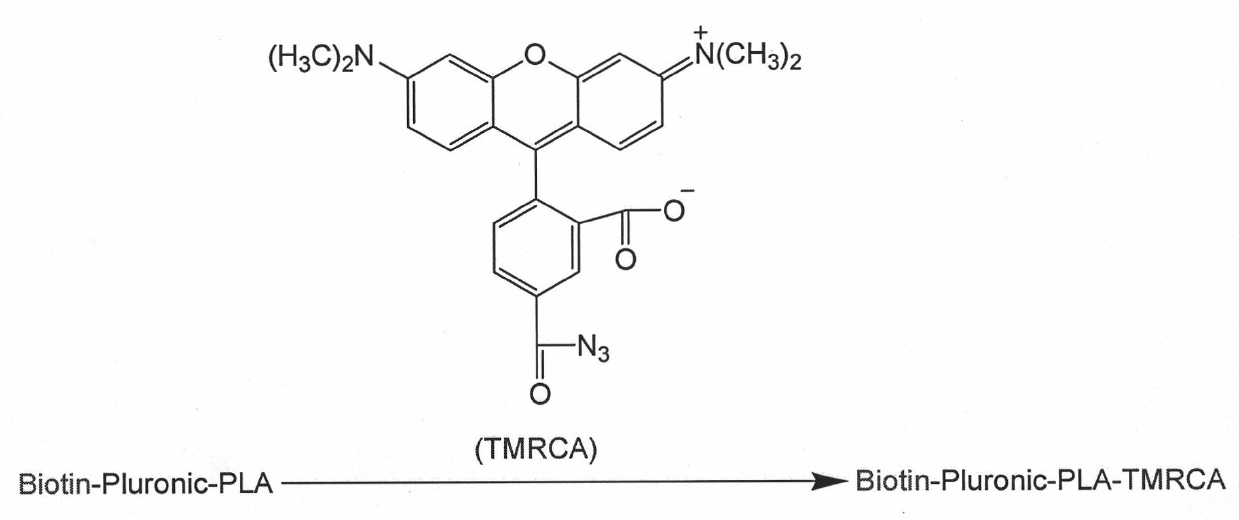
[0051] Synthesis of Biotin-Pluronic-PLA-TMRCA.
[0052] a) Synthesis of Biotin-Pluronic-OH: Feed according to Pluronic:Biotin molar ratio 1:1.2, dissolve in dichloromethane, and add 4-dimethylaminopyridine, then add dropwise the solution in dichloromethane under ice-water bath conditions 1,3-Dicyclohexylcarbodiimide. After the dropwise addition, the reaction was continued at room temperature for 38 hours. Then with 10% NaHCO 3 The entire reaction solution was extracted to remove unreacted Biotin. After the extraction, the reaction solution was frozen overnight, and then filtered to remove insoluble matter. Then the reaction solution was concentrated, then dropped into cold anhydrous ether, filtered, and vacuum-dried to obtain Biotin-Pluronic-OH with one end of Biotin modified. It may contain a small amount of Biotin-Pluronic-Biotin with modified Biotin at both ends, and this polymer can be purified and removed in the reaction of step b).
[0053] b) Synthesis of Biotin-Pl...
Embodiment 2
[0056] Application of Biotin-Pluronic-PLA for targeting of cancer cells.
[0057] (1) Biotin-Pluronic-PLA nanoparticles are first prepared by a conventional method, and the nanoparticles can be prepared by dialysis. The operation steps are as follows: first dissolve 10 mg of Biotin-Pluronic-PLA copolymer in tetrahydrofuran (THF), and then add the solution dropwise into 12.5 g of ultrapure water while stirring. The formed Biotin-Pluronic-PLA nanoparticles were dialyzed in water to remove THF;
[0058] (2) Embedding the anticancer drug Paclitaxel in the nanoparticles formed by the copolymer, first dissolve Biotin-Pluronic-PLA-TMRCA 24mg and Paclitaxel 1.5mg in THF, and then add the solution dropwise to 45g of ultrapure water under stirring middle. The formed Paclitaxel-entrapped Biotin-Pluronic-PLA nanoparticles were dialyzed in water to remove THF and non-entrapped paclitaxel.
[0059] (3) The targeting of Biotin-Pluronic-PLA nanoparticles to cancer cells is realized by a th...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com