Glaucocalyxin A acid ester derivative as well as preparation method and application of Glaucocalyxin A acid ester derivative
A technology of cylinate and cyanin, which is applied in the field of cyanin derivatives, can solve the problems of fast elimination, short half-life, and low polarity of GLA, and achieve a good inhibitory effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0083] Embodiment 1: the preparation of cyanine A
[0084] Using fragrant tea herbs (aerial parts) as raw materials, through the steps of extraction, solvent treatment, resin treatment and recrystallization, to prepare cyanine (GLA), the specific process is as follows:
[0085] Step 1 is an extraction step, which further includes:
[0086] Step 1.1: Take fragrant tea and vegetable medicinal materials (aerial parts) and grind them to 20-50 mesh;
[0087] Step 1.2: Mix the obtained pulverized product with 95% ethanol (A.R.) at a volume ratio of 1:5 to 1:15, heat, reflux at 80°C to 90°C for 1 to 2 hours, extract and filter to obtain the extract liquids and residues;
[0088] Step 1.3: Mix the residue obtained in step 1.2 with 95% ethanol (A.R.) at a volume ratio of 1:5 to 1:15, heat, reflux at 80°C to 90°C for 1 to 2 hours, extract and filter, To obtain the extract and the residue, repeat this step 1 to 3 times;
[0089] Step 1.4: Combine the extracts obtained in steps 1.2 an...
Embodiment 2
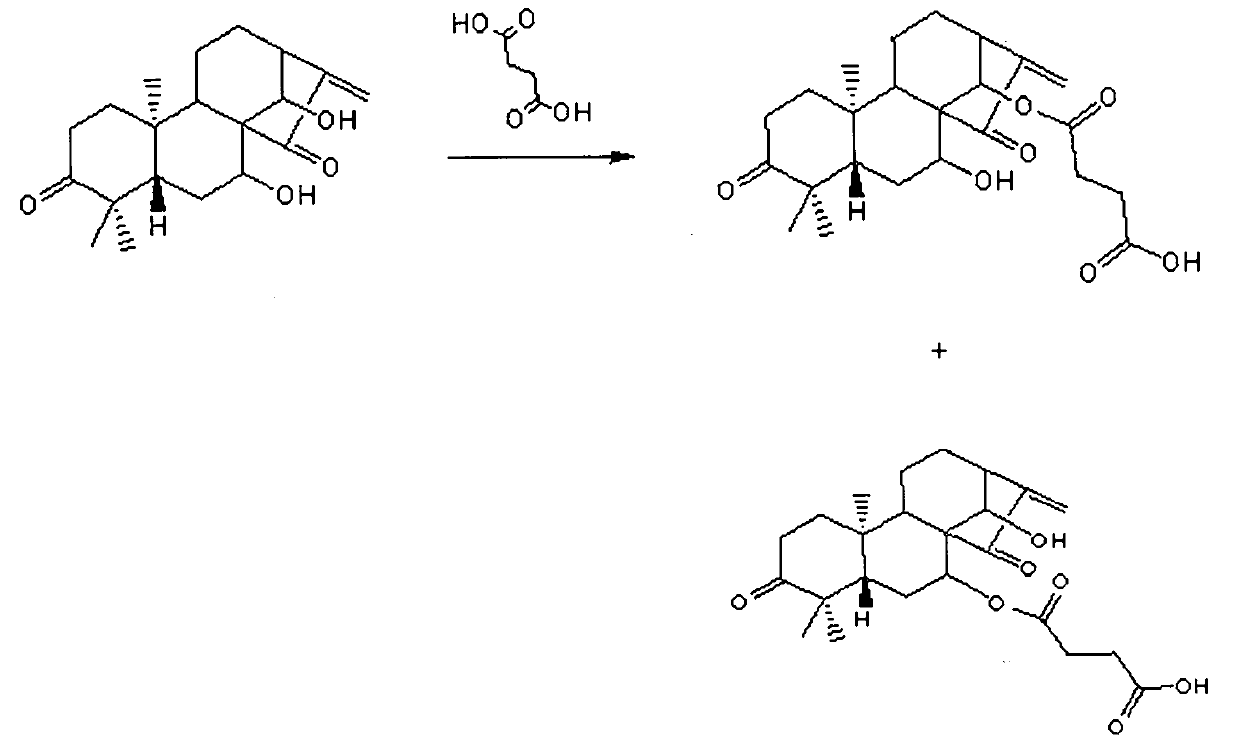
[0107] Example 2: Derivatization of succinic acid
[0108] according to figure 1 In the reaction equation shown, the cyanine A prepared in Example 1 is derivatized with succinic acid, thereby introducing a modified substituent into the cyanine A to obtain the succinate of cyanine A, The derivatization step 5 further comprises:
[0109] Step 5.1: Dissolve 0.96-1.16 g of succinin obtained in step 4 in 25-35 ml of tetrahydrofuran, and add 0.857-1.057 g of succinic acid and 0.067-0.087 g of DMAP in turn at room temperature, and stir the reaction at room temperature 10-14 hours;
[0110] Step 5.2: heating the reaction solution in step 5.1 to 40°C-50°C and distilling under reduced pressure to remove tetrahydrofuran;
[0111] Step 5.3: add 25-35ml of water to the reaction solution in step 5.2 to dilute, then extract 2-4 times with 25-35ml of ethyl acetate, combine the organic phases, wash with saturated brine, and dry over anhydrous sodium sulfate;
[0112] Step 5.4: Heat the was...
Embodiment 3
[0114] Example 3: Glycine derivatization
[0115] according to Figure 3a~3b As shown in the reaction equation, the succinate of cyanine A prepared in Example 2 is derivatized with glycine, thereby introducing a modified substituent into cyanine A to obtain the succinate of cyanine A. Derivatives of acid ester and glycine, the derivatization step 6 further comprises:
[0116] Step 6.1: Dissolve 113.5-133.5 mg of succinate of cerulein in 1-5 ml of tetrahydrofuran (THF), and dissolve 19.3-21.3 mg of N-hydroxysuccinimide (NHS) in 3-7 ml of phosphate buffer solution (PBS buffer solution);
[0117] The PBS buffer solution is 0.2 μmol / L and its pH=8.0;
[0118] Step 6.2: Add the obtained PBS solution of NHS dropwise to the THF solution of succinate A succinate at room temperature, stir and react at room temperature for 10 to 20 minutes, and then heat the obtained reaction solution to 30°C to 40°C °C, distilled under reduced pressure to remove THF to obtain a yellow oily crude pr...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com