Crystal form of benzoylaminopyridine derivative, and application thereof
A technology of crystal forms and diffraction peaks, which can be applied to medical preparations containing active ingredients, drug combinations, digestive systems, etc., and can solve problems such as undisclosed crystal structures and undisclosed compound microstructures.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
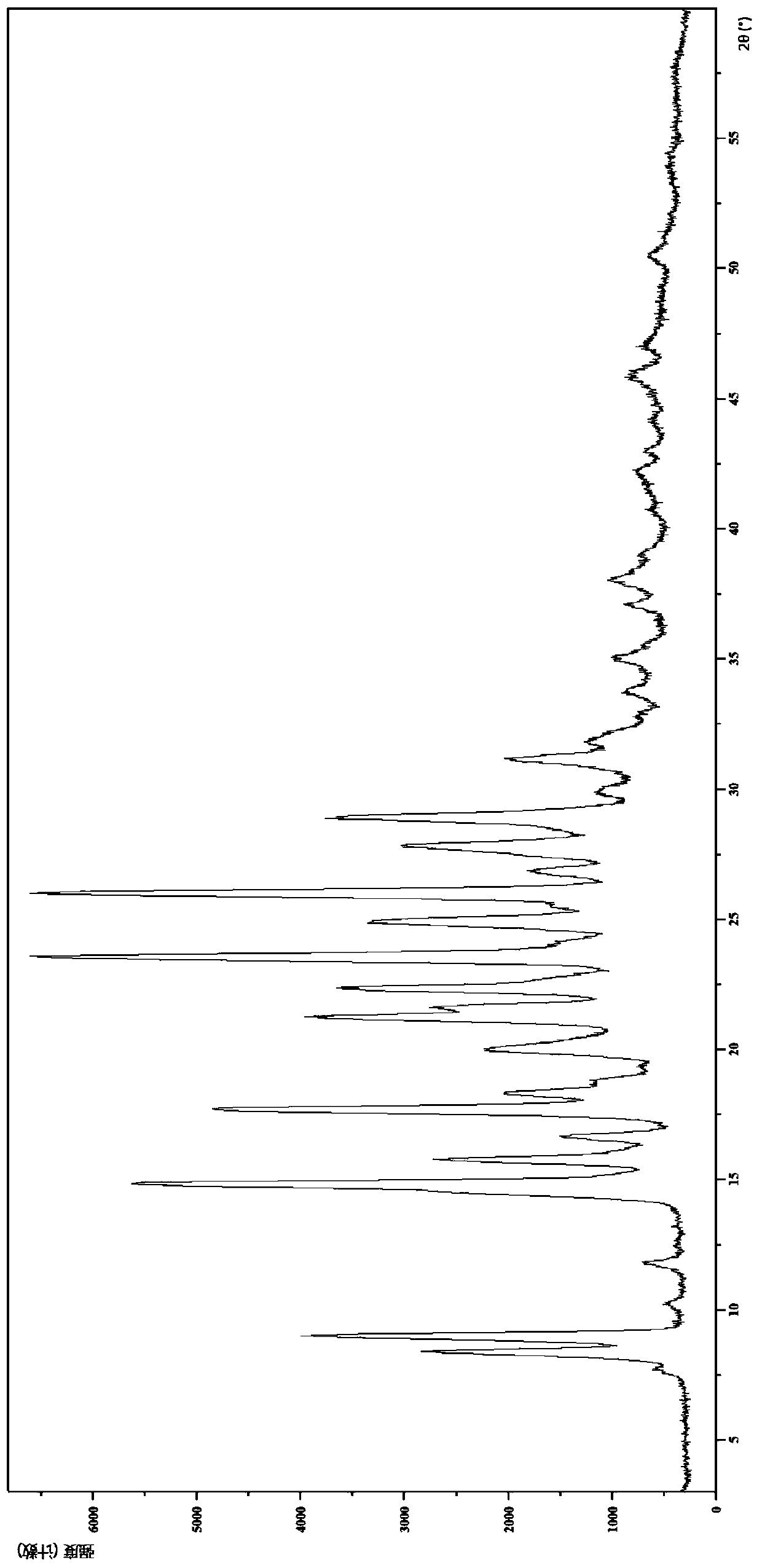
[0133] Embodiment 1 The crystal form I of the compound of formula (I)
[0134] 1. Preparation of Form I
[0135] Compound 5-(4-cyclopropyl-1H-imidazol-1-yl)-N-(6-(6,7-dihydro-5H-pyrrolo[2,1-c][1,2 ,4] Triazol-3-yl)pyridin-2-yl)-2-fluoro-4-methylbenzamide (506mg) was added into dichloromethane (10.0mL) and stirred to dissolve, then absolute ethanol was added dropwise (5.0 mL) was stirred for 5 minutes, allowed to stand at room temperature for 3 days, filtered with suction, and the filter cake was vacuum-dried at 60° C. to obtain a white solid (472 mg, 93.28%).
[0136] 2. Identification of Form I
[0137] (1) Analysis and identification by Empyrean X-ray powder diffraction (XRPD): using Cu-Kα radiation, with the following characteristic peaks expressed in angle 2θ: 7.66°, 8.35°, 8.95°, 10.20°, 11.76°, 14.51°, 14.82 °,15.74°,16.61°,17.60°,17.73°,18.28°,18.76°,20.02°,21.20°,21.59°,22.32,23.51°,24.87°,25.96°,26.80°,27.74°,28.86°,29.89 °, 31.09°, 31.86°, 32.82°, 33.72°, 35....
Embodiment 2
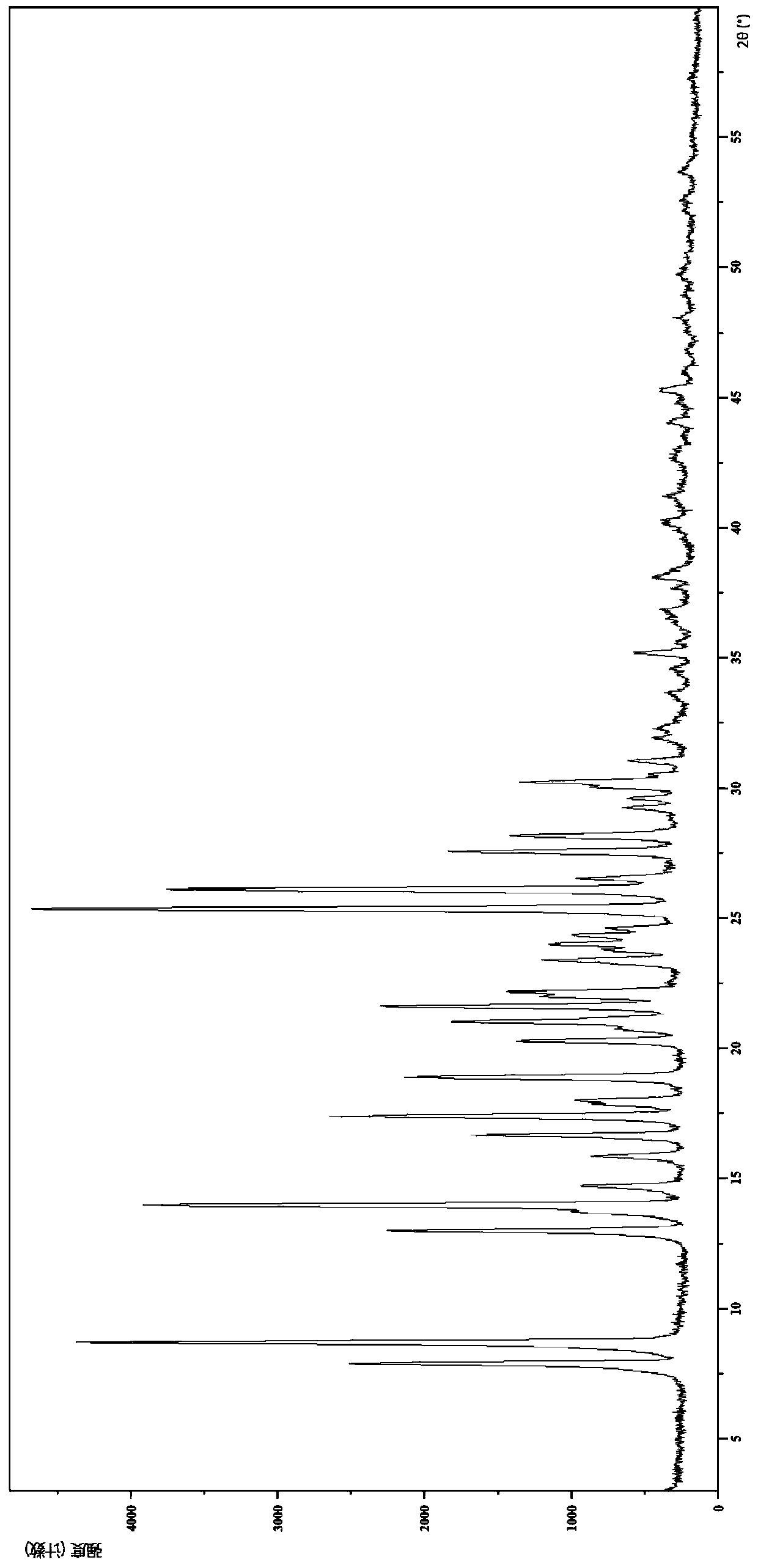
[0139] Embodiment 2 The crystal form II of the compound of formula (I)
[0140] 1. Preparation of Form II
[0141] The compound 5-(4-cyclopropyl-1H-imidazol-1-yl)-N-(6-(6,7-dihydro-5H-pyrrolo[2,1-c][1,2,4 ]triazol-3-yl)pyridin-2-yl)-2-fluoro-4-methylbenzamide (1.27g) was added in acetone (20.0mL), beaten at room temperature for 7.5 hours, suction filtered, and the filter cake was Drying in vacuo afforded a white solid (1.16 g, 91.34%).
[0142] 2. Identification of Form II
[0143] (1) Analysis and identification by Empyrean X-ray powder diffraction (XRPD): using Cu-Kα radiation, with the following characteristic peaks expressed in angle 2θ: 7.85°, 8.66°, 12.95°, 13.72°, 13.95°, 14.66°, 15.82 °,16.62°,17.36°,17.81°,17.98°,18.85°,20.25°,20.69°,21.00°,21.57°,21.95°,22.14°,23.33°,23.73°,23.97°,24.30°,24.57°, 25.32°, 26.06°, 26.49°, 27.52°, 28.12°, 29.20°, 29.56°, 30.01°, 30.20°, 30.48°, 31.00°, 31.87°, 32.25°, 33.56°, 34.49°, 35.14°, 36.64° , 37.61°, 38.11°, 40.13°, 41.1...
Embodiment 3
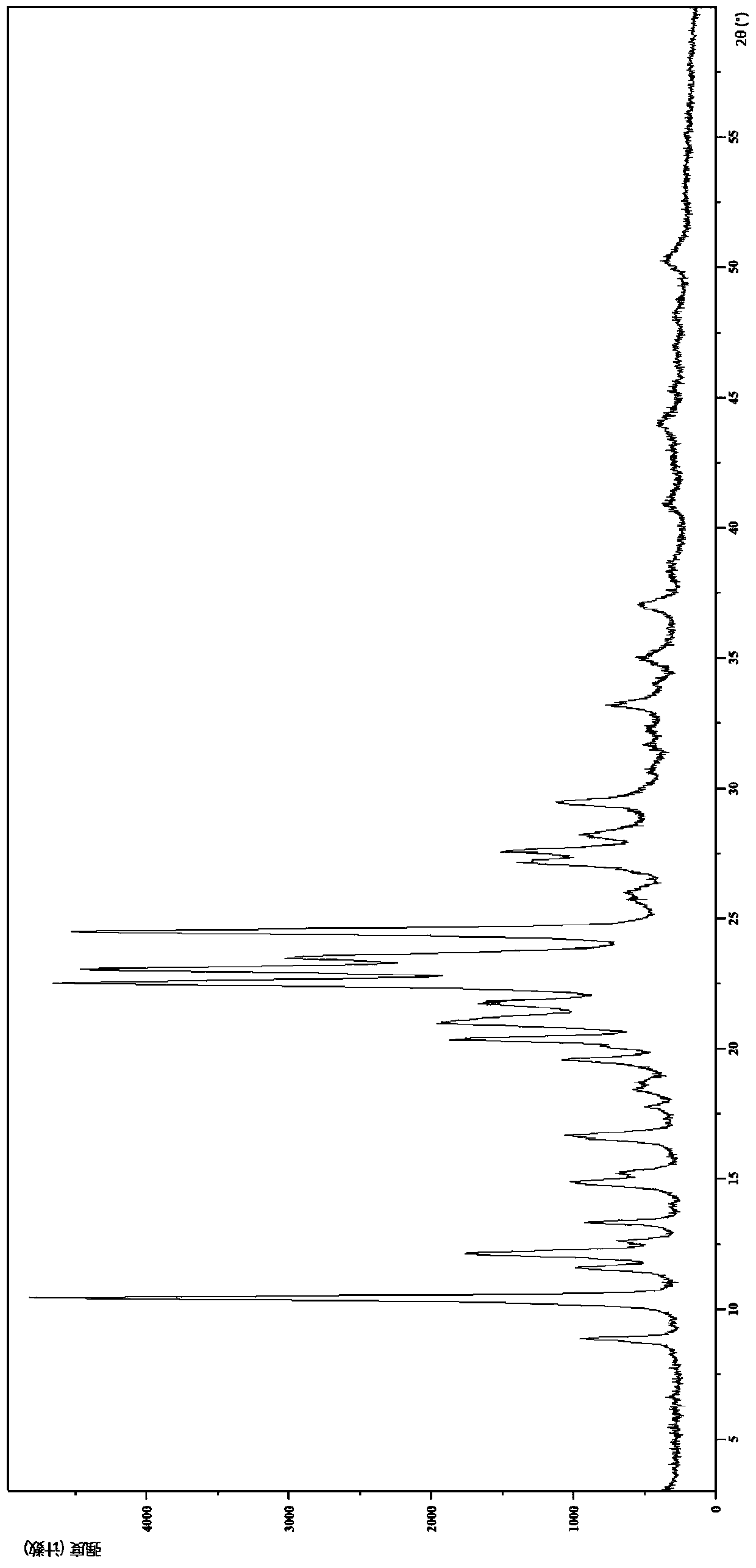
[0145] Embodiment 3 The crystal form III of the compound of formula (I)
[0146] 1. Preparation of Form III
[0147] The compound 5-(4-cyclopropyl-1H-imidazol-1-yl)-N-(6-(6,7-dihydro-5H-pyrrolo[2,1-c][1,2,4 ]triazol-3-yl)pyridin-2-yl)-2-fluoro-4-methylbenzamide (551mg) was added to methanol (11.0mL), beaten at room temperature for 17 hours, suction filtered, and the filter cake was vacuum-dried at room temperature , to obtain a white solid (411 mg, 74.59%).
[0148] 2. Identification of Form III
[0149] (1) Analysis and identification by Empyrean X-ray powder diffraction (XRPD): using Cu-Kα radiation, it has the following characteristic peaks expressed in angle 2θ: 8.82°, 10.38°, 11.55°, 12.10°, 12.58°, 13.30°, 14.83 °,15.22°,16.60°,17.73°,18.47°,19.53°,20.32°,21.03°,21.70°,22.49°,23.01°,23.47°,24.45°,25.88°,27.13°,27.55°,28.19°, 29.43°, 31.67°, 33.18°, 34.99°, 36.99°, 38.45°, 40.94°, 43.95°, 45.32°, 48.08° and 50.28°, there is an error tolerance of ±0.2°.
[0150] (...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com